
Microbiome Based Therapeutics Regulatory Landscape
Regulatory Landscape - Overview
Microbiome based therapeutics Regulatory Landscape: Product Overview
Microbiome based therapeutics are developed with the intension to modulate the human microbiome to maintain and restore health. Traditional drugs usually target the pathogens or cells directly, but microbiome-based therapeutics aim to influence only microbial communities in the body primarily gut microbiome and skin, lungs and other mucosal sites.
Microbiome based therapeutics Applications:
Fecal Microbiota Transplantation (FMT) is a key application of microbiome-based therapeutics, where fecal matter from a healthy donor is introduced into a recipient’s gastrointestinal tract to restore microbial balance. This is most often accomplished using colonoscopies, enemas, or capsules, and is primarily used to treat recurrent Clostridioides difficile infections, with growing potential in other microbiome-related disorders.
Probiotics, Prebiotics, and Synbiotics are widely used to support gut health. Probiotics are live beneficial microbes that help balance the gut microbiota, while prebiotics are non-digestible fibers that feed these good bacteria. Synbiotics combine both to enhance their effects.
Next-generation probiotics (NGPs), such as Faecalibacterium prausnitzii and Akkermansia muciniphila, offer targeted benefits like reducing inflammation with potential roles in managing chronic diseases such as inflammatory bowel disease and metabolic disorders, NGP strains may influence the gut–brain axis, supporting mental health. Additionally, NGPs are being explored for personalized probiotic therapies, synthetic biology applications, and targeted delivery methods, showcasing their potential in precision medicine.
Microbial Metabolite-Based Therapies utilize small molecules produced by gut bacteria—such as short-chain fatty acids (SCFAs), bile acids, and other signaling compounds—to influence host health. These metabolites play key roles in regulating immune responses, reducing inflammation, and supporting metabolic functions. For example, SCFAs like butyrate help strengthen the gut barrier and have anti-inflammatory effects. Such therapies are being explored for treating neurological, metabolic, and inflammatory bowel diseases, with ongoing research aimed at integrating them into clinical practice. Notably, butyrate enhances gut barrier integrity, modulates immune activity, and exhibits neuroprotective effects, making it a promising candidate for treating neurodegenerative diseases like Alzheimer’s and Parkinson’s.
Microbiome based therapeutics Product Development steps:
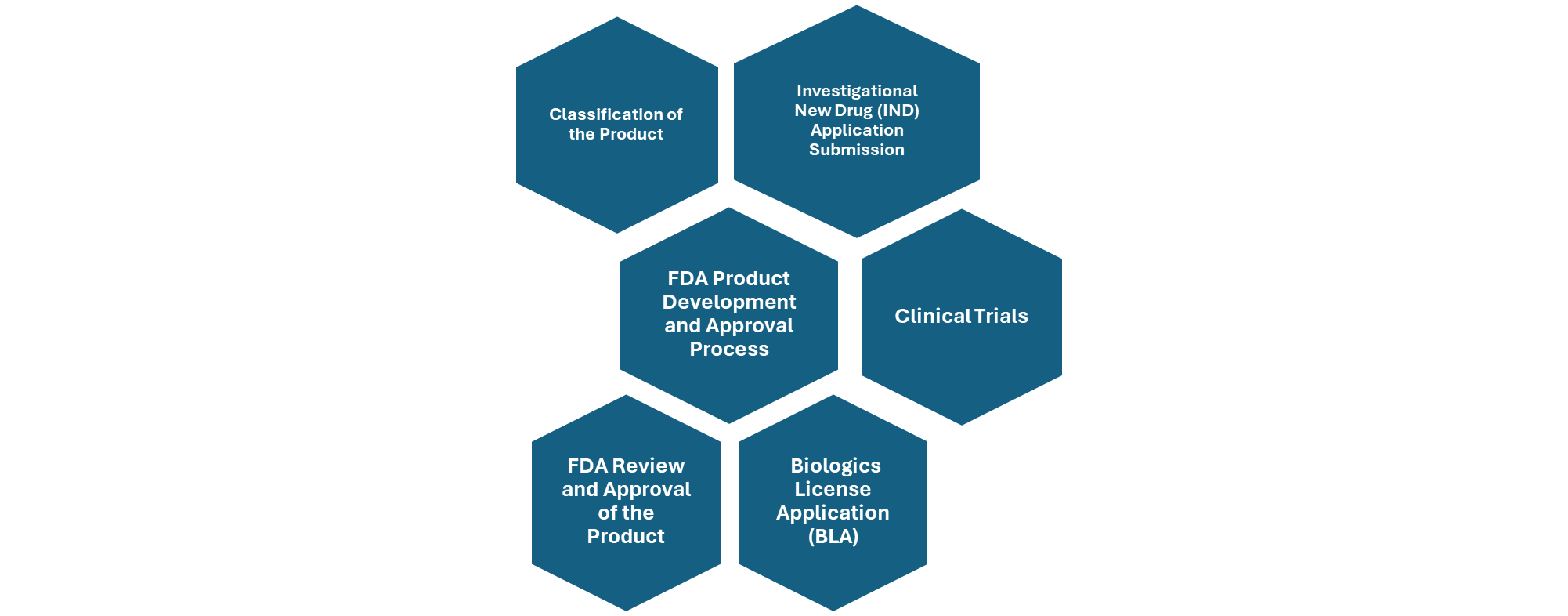
Figure: Overview of FDA Product Development and Approval Process.
Microbiome based therapeutics Market Size Overview:
As per MRFR analysis, the Microbiome Therapeutic Market Size was estimated at 5.02 (USD Billion) in 2023. The Microbiome Therapeutic Market Industry is expected to grow from 5.41(USD Billion) in 2024 to 12.5 (USD Billion) by 2035. The Microbiome Therapeutic Market CAGR (growth rate) is expected to be around 7.91% during the forecast period (2025 - 2035).
Microbiome based therapeutics Regulatory Landscape:
There are several key regulatory agencies who oversee the approval and monitoring of Microbiome based therapeutics to ensure their safety, efficacy, and quality.
|
Regulatory agencies |
Regulatory Ministry |
|
Federal Food and Drug Administration |
United States: Department of Health and Human Services (HHS) |
|
The Medicines and Healthcare products Regulatory Agency |
United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
|
Central Drug Standard Control Organization |
India: The Ministry of Health and Family Welfare |
|
South African Health Products Regulatory Authority (SAHPRA) |
National Department of Health. |
|
Pharmaceuticals and Medical Devices Agency (PMDA) |
Japan: Ministry of Health, Labour and Welfare. |
|
National Medical Products Administration (NMPA) |
China: The Ministry of Health |
|
Health Sciences Authority |
Singapore: The Ministry of Health |
|
European Medicine Agency |
European union |
|
Brazilian Health Regulatory Agency (Anvisa) |
Ministry of Health, part of the Brazilian National Health System (SUS) |
Microbiome based therapeutics Guidelines:
Microbiome based therapies are given to individuals with medical conditions linked to gut dysbiosis or an imbalance in the body’s microbial community, which aim to restore the healthy microbiota composition and function, for instance, it is administered to patients with recurrent Clostridioides difficile infection (rCDI) or individuals with inflammatory Bowel Disease (IBD) or to patients suffering with metabolic disorders.
Fecal Microbiota Transplantation (FMT) is considered an investigational treatment by the FDA, primarily used for patients with recurrent C. difficile infections, and for its administration patients informed consent is required. FMT is typically safe, but the method of delivery, often through endoscopy, can cause short-term side effects like bloating, gas, constipation, or mild diarrhea, which are usually temporary and resolved on their own.
Microbiome based therapeutics Classification of the Product:
Microbiome based therapeutics Regulatory Process Overview, By Country:
The regulatory aspects of microbiome-based therapies are complex and evolving. As these therapies represent a relatively new field, regulatory frameworks are still being developed and refined.
The regulatory pathway for microbiome-based therapeutics involves several structured steps to ensure safety, efficacy, and quality. Here are some key regulatory considerations for microbiome-based therapies:
Classification of the product: First, the product must be classified appropriately—either as a drug, biologic, medical device, or combination product—based on its composition and mechanism of action. This classification determines the regulatory framework it will follow. In some cases, such as Fecal Microbiota Transplantation (FMT), regulatory agencies like the U.S. FDA may exercise enforcement discretion, allowing limited use without full approval under specific conditions.
Safety and risk assessment: Once classified, the product must undergo rigorous clinical trials, starting with preclinical studies and progressing through Phase I (safety), Phase II (efficacy and dosing), Phase III (confirmation of efficacy and monitoring of adverse effects), and Phase IV (post-marketing surveillance). Throughout this process, developers must conduct thorough risk assessments, particularly for live microbial products, to evaluate potential infection risks and immune responses.
Manufacturing and quality control: Manufacturing must comply with Good Manufacturing Practices (GMP) to ensure consistency, purity, and potency of the product. These steps collectively form the regulatory backbone for bringing microbiome-based therapies to market. Product Development should follow standardized protocols for collection, processing, and storage of microbiome samples for ensuring product consistency and safety.
Regulatory submissions required by FDA during therapeutic approval process include:
- Investigational New Drug (IND) Applications are required for most microbiome-based therapies before starting clinical trials. IND application should include treatment protocol including an informed consent of the participants involved in clinical trials. The FDA reviews preclinical data, manufacturing details, and clinical plans to ensure participant safety.
- If the therapy is classified as a biological product, a Biologics License Application (BLA) must be submitted for FDA approval, including detailed data on safety, effectiveness, and production methods.
- For Fecal Microbiota Transplantation (FMT) used to treat Clostridium difficile infections unresponsive to standard treatments, the FDA currently allows its use under enforcement discretion, meaning full regulatory approval is not required in these specific cases.
- However, for other uses of FMT, an IND application is still necessary to proceed with clinical research.
EMA Regulatory Requirements for approval of Microbiome based Therapeutics include as follows:
Microbiome-based therapies are classified as Advanced Therapy Medicinal Products (ATMPs), which even comprise gene therapy, somatic cell therapy, and tissue-engineered products. These therapies are complex in nature and therefore they follow special regulatory pathways.
Clinical trials in the EU, companies must obtain a Clinical Trial Authorization (CTA), similar to the FDA’s IND, which involves a detailed review of preclinical data, clinical plans, and manufacturing processes.
International Collaboration: The FDA and EMA also work together through international collaborations to align regulatory standards. Organizations like the International Council for Harmonization (ICH) help create shared guidelines to support global development of microbiome-based therapies.
Microbiome based therapeutics updates:
May 2023, The U.S. FDA has approved Vowst (formerly SER-109), developed by Seres Therapeutics and Nestlé, as the second microbiome-based therapy for preventing recurrence of Clostridioides difficile infection (CDI) after antibiotic treatment. Vowst is an oral capsule containing spore-forming Firmicute bacteria purified from healthy donors. It follows the approval of Rebyota, a rectally administered microbiota-based product by Ferring/Rebiotix. CDI is a serious healthcare-associated infection causing up to 30,000 deaths annually in the U.S. Traditional antibiotics often fail to prevent recurrence, as they disrupt both harmful and beneficial gut bacteria. Microbiome-based therapies like Vowst aim to restore healthy gut flora to prevent reinfection.
In the ECOSPOR III Phase III trial, 88% of Vowst recipients had a sustained clinical response at 8 weeks, compared to 60% in the placebo group. The ECOSPOR IV trial, an open-label study with 263 patients, further supported its safety and efficacy. Common side effects included bloating, fatigue, constipation, chills, and diarrhea. Vowst was approved under Priority Review, Orphan and Breakthrough Therapy Designation.
November 2022, two microbiome-based therapies, BIOMICTRA by BiomeBank and REBYOTA by Ferring Pharmaceuticals, were approved for treating recurrent Clostridioides difficile infection, a condition marked by severe diarrhea and significant unmet medical need. BIOMICTRA is a whole microbiota transplant, while REBYOTA is a live biotherapeutic, reflecting the diversity in current microbiome drug approaches.
In 2022, there were 288 new live biotherapeutic assets and 199 whole ecosystem-derived products announced, 526 clinical trials were identified in the microbiome therapeutics field, as of February 2023, showing a 25% average annual growth rate since 2012.
Microbiome based therapeutics Regulatory Challenges and possible risk in development:
The development of microbiome-based therapeutics faces several clinical and manufacturing challenges. During Clinical trials, Patient selection is complex, particularly in advanced trial phases, and designing effective, standardized trials remains difficult.
Regulatory uncertainty makes approval of these therapeutics furthermore challenging. Many companies depend on external experts, raising concerns about the need for internal capabilities to ensure long-term success.
Additionally, planning for scale-up during development is still an evolving process. On the manufacturing side, ensuring product stability and self-life for live biotherapeutic products is a major hurdle.
Regulatory frameworks are still evolving, with agencies like the FDA refining their guidance, and the lack of industry benchmarks makes it difficult for companies to navigate development confidently.
Microbiome based therapeutics Competitive Landscape Dashboard:
Companies With Marketed Microbiome based therapeutics:
- Synlogic
- Amgen
- Ferring Pharmaceuticals
- Mikrobio
- Havencrest Capital
- Medosome Biotec
- Zymeworks
- 2seventy bio
- AOBiome
- Eden Biosciences
- AbbVie
- Diverse Biotech
- Microbiotica
- Enterome
- Seres Therapeutics
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”