
Regulatory Landscape - Overview
Ayurvedic Products Drugs Regulatory Landscape: Product Overview
Ayurveda, the ancient Indian system of medicine, focuses on achieving balance in the body, mind, and spirit through natural remedies and holistic practices. Healthcare and consumer care Ayurveda products include a variety of herbal supplements like adaptogens (e.g., Ashwagandha), digestive aids (e.g.,Triphala), and essential oils for aromatherapy. Skincare offerings feature herbal creams and oils infused with neem and turmeric, while personal care products include hair oils and herbal toothpastes. Ayurvedic food and beverages, such as herbal teas and superfoods like ghee, align with dietary principles for overall wellness. Increasingly, consumers are drawn to sustainable, natural, and customizable remedies tailored to their unique dosha types (Vata, Pitta, Kapha). Regulatory authorities involved ayurvedic product approval to ensure its safety and efficacy is Ministry of Ayush, under Central Drugs Standard Control Organisation (CDSCO). According to the research study published by Ayush, around 25% of ayurvedic and herbal products were exported from India in 2023 in the U.S. market space.Ayurvedic Product types
Form/ ingredients used
Ayurvedic products mode of action
- Asparagus racemosus wild is also known as Shatavari is a potent ayurvedic product having rejuvenation effect- it can supply hormones to females, mainly suggested for those who have hysterectomies. It also has the ability to strengthen immune system, maintain urinary tract, and it can also purify blood.
- Commiphora Mukul Engl. (i.e. Guggul) is the major ayurvedic ingredient in the therapeutics of joint and immunocare. It works by increasing the count of WBCs to provide strong immune modulating properties, it can also show therapeutic effect for common cold, and many other health diseases, it can also lower the levels of cholesterol, Trglycerides.
- Cypes scariosus Br. (i.e. Nagarmusta) is beneficial for maintaining healthy genitourinary system and also show hepatoprotective properties.
Applications of Ayurvedic products
- Ayurveda offers a range of tablets and capsules designed to promote overall health and address specific wellness needs. Common formulations include digestive aids like Triphala, which helps detoxify and improve digestion, and stress-relievers like Ashwagandha, known for its calming properties. For joint health, Boswellia and Turmeric are popular due to their anti-inflammatory benefits. Immune support can be enhanced with Amla capsules, rich in vitamin C and antioxidants, while Neem tablets are often used for skin health due to their purifying effects.
- Ayurvedic creams are typically designed to address specific skin concerns, such as dryness, aging, or uneven tone, while serums may focus on delivering concentrated nutrients and antioxidants deep into the skin. The use of natural ingredients allows these products to be gentle yet effective, making them suitable for a variety of skin types, including sensitive skin.
- Ayurvedic face washes are formulated to cleanse the skin gently while harnessing the power of natural ingredients known for their therapeutic properties. Key components often include neem, recognized for its antibacterial and acne-fighting benefits; turmeric, celebrated for its anti-inflammatory and brightening effects; and sandalwood, which soothes and hydrates the skin.
Ayurvedic Products Development Steps:
Traditional Ayurvedic Drug development process includes following main steps:- Bioactive compounds isolation and its synthetic modification
- Safety and effectiveness evaluation
- If new drug is developed, then getting regulatory approval for it
- Undertaking clinical trials for the new drug
- Survey of disease prevalence and drug formulation: on basis of proper literature review, ingredients previous clinical data, classical evidence
| Drug development phase | Step details |
| Phase 2 | Collection of raw drugs (on basis of ayurvedic textual methods, good agricultural and field collection practices) |
| Phase 3 | Botanical, chemical study of drug ingredients or pharmacogenetic (Based on guidelines available and classical methods) |
| Phase 4 | SOP formulation, performing standardisation, study of stability, checking quality of drug- (On basis of available physical, chemical and biological parameters which are used for standardisation, based on classical methods) |
| Phase 5 | Pre-clinical study to ensure safety of drug (includes acute, subacute, chronic study as per use of drug)- (Using suitable animal models with appropriate animal ethical clearance as per guidelines) |
| Phase 6 | Animal studies to check effectiveness and biological activity of drugs- (Study of mechanism of action for clinical corelation) |
| Phase 7 | Study design and clinical process formulation- (Based on clinical guidelines) |
| Phase 8 | Clinical trial execution- (Includes IEC/IRB and CTRI registration approval Conducting, monitoring, coordinating trial, Analysis of generated data, Publication) |
Ayurvedic Products Market Size Overview:
As per MRFR analysis, the Ayurvedic product Market Size was estimated at 16,822.71 (USD Million) in 2024. The Ayurvedic Products Market Industry is expected to grow from 19,452.62 (USD Million) in 2025 to 88,872.15 (USD Million) till 2035, at a CAGR (growth rate) is expected to be around 16.41% during the forecast period (2025 - 2035). growing awareness of health and wellness benefits associated with ayurveda, increasing government support for Ayurveda Products, and growing adoption for ayurvedic products across the globe are the key market drivers enhancing the growth of the market.
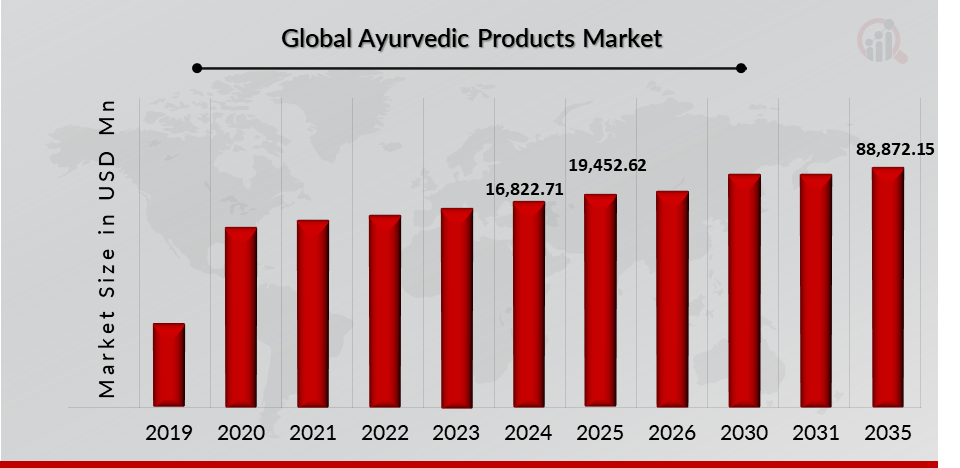
Source: The Secondary Research, Primary Research, MRFR Database and Analyst Review
Ayurvedic Products Regulatory Landscape:
Ayurvedic pProducts Guidelines: Eligibility:
Ayurveda offers traditional and natural treatment options, so it is generally considered safe treatment option for broad population, they are often used as an alternative to conventional products, people prefer ayurveda for some benefits like no side effects, natural healing, they even support conventional treatment. For example, most of the cancer patients in India use ayurvedic medicines as an immune booster along with their oral drugs or other cancer treatment medications which help them fight with cancer more effectively.Dosage:
Ayurvedic Products Classification of The Product: Ayurvedic Products Regulatory Process Overview, By Country:
Ministry of Ayush under central drug standard control organization (CDSCO) is responsible for development, education, research, in the areas of ayurveda, yoga, naturopathy, Unani, siddha and Homeopathy traditional therapeutics. The drugs and cosmetic act 1940 and drugs and cosmetic rules 1945, has all the guidelines for the Ayurvedic, Siddha, Unani (ASU) drugs, manufacturers must comply with these drugs to ensure product quality, efficacy and safety. There is Drug Policy Section (DPS) under Ministry of Ayush which looks after regulatory and quality control functions for ayurveda also is involved in implementing drug related initiatives. This section act as central drug control framework of ASU&H drugs, which coordinates with state licensing authorities/drug controllers also with ayurvedic drug manufacturers to provide regulatory guidance, clarification, direction, legal provisions for development of ayurvedic products. Government of India has formed 2 organizations namely Pharmacopoeia Commission for Indian Medicine and Homeopathy (PCIM&H) an autonomous body and pharmacopoeia Laboratory for Indian Medicine (PLIM) for Pharmacopoeia standardization of Ayurvedic drugs. They have also issued 2 voluntary certification schemes for ayurvedic products, which includes following:- Quality Council of India (QCI) Certification scheme, which is an autonomous body
- For ayurvedic products which comply with domestic regulations, will get “AYUSH Standards Mark”.
- For ayurvedic products which comply with international norms, will get “AYUSH Premium Mark”.
- Certificate of Pharmaceutical Products (CoPP) for Ayurveda product, which is issued by Drug Controller General of India (DCGI) under CDER from CDSCO.
Ayurvedic Products who have got COPP grant as per WHO-GMP Certification scheme of CDER
- Anantavati Tablet
- Nokamen Tablet
- Senadexin Ananta Tablet
- Artrex Tablets
- Artovid-20 Tablets
Ayurvedic Products Regulatory Updates and Amendment’s:
US FDA in 2023, discovered safety issues with some of the ayurvedic products example they found concerning levels of heavy metals in ayurvedic products exported to U.S. market such as lead, mercury, arsenic, whether it is a ingredient of the age old ayurvedic formula or as a contamination in product it will cause poisoning risk in consumers, and their presence was not disclosed on the labels, US FDA has warned that no ayurvedic drug is approved by them and consumers should consider the risk of taking it. FDA says it cannot ensure its safety. In 2023, the FDA India Office co-hosted training for growers and producers of spices, herbs, and botanicals. The training focused on Good Agricultural Practices (GAPs) and Good Manufacturing Practice (GMP) requirements to ensure the safe production of botanical products. In the year 2022 Food Safety and Standards Authority of India (FSSAI), consulting with Ministry of Ayush has issued Food Safety and Standards (Ayurveda Aahara) Regulations, which are notified in official gazette on 5th May 2022, including the regulations of addition of vitamins and minerals and covering health claims.- Regulations state that no manufacturing or selling of Ayurveda Aahara for infants up to age 24 months by the companies. And no addition of vitamins, minerals, amino acids in Ayurveda Aahara, only if vitamins, minerals are naturally present in product they are allowed and can be mentioned on labels.
- Products covered under this should only include the approved ingredients, and some of which include guar Arabic, pectin, konjac flour, honey, date syrup, curcumin, turmeric, rose oil, rosemary oil.
- In addition, they have rule which state that the labelling used for product or its presentation and advertisement of Ayurveda Ahara should not claim to prevent, treat or cure human disease or refer such properties. Label should include target consumer group and intended purpose, duration of use and other specific information
Ayurvedic Products Regulatory Challenges:
Strict safety and quality standards pose significant challenges to the growth of the Ayurvedic products market. Stringent regulatory guidelines for product approval require rigorous testing and scientific evaluation by authorities, focusing on purity, ingredient integrity, and manufacturing processes. In the United States, the Food and Drug Administration (FDA) also plays a crucial role in regulating Ayurvedic products, ensuring they meet safety and efficacy standards. As well, manufacturing companies cannot get license approval for the ayurvedic products until they are approved by the Food Safety and Standards Authority of India (FSSAI). For instance, in May 2022, the Ministry of AYUSH and the Food Safety and Standards Authority of India (FSSAI), both under the Ministry of Health and Family Welfare, Government of India (MoHFW), prepared regulations for safety and quality standards for food products for "Ayurveda Aahara" category. Such stringent regulatory measures are significantly restricting market growth due to the complex requirements involved in the manufacturing and licensing processes for Ayurvedic products. The extensive testing and scientific evaluations mandated by regulatory authorities necessitate a considerable investment of time and resources. Manufacturers must navigate intricate protocols to ensure compliance with safety, quality, and efficacy standards, which can vary by region and regulatory body. Like conventional therapeutics, ayurvedic medicines don’t have extensive clinical trials to validate their efficacy and safety. In India Ayush and CDSCO looks after approval and regulation of ayurvedic products but to take this in harmony with global standards like FDA is a challenge. Ayurveda provides traditional therapeutics and other products widely accepted in India, but its global acceptance is a challenge.
Possible Risk in development of Ayurvedic Products
Ayurvedic Products Competitive Landscape Dashboard: Companies With Marketed Ayurvedic Products Products
- Dabur Ltd.
- Patanjali Ayurved Limited
- Himalaya Wellness Company
- Maharishi Ayurveda
- Lotus Herbals
- Emami Limited
- Baidyanath
- Hamdard
- Vicco Laboratories
- Kerala Ayurveda Limited
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”