
Regulatory Landscape - Overview
CBD Oil Regulatory Landscape: Product Overview
CBD is cannabidiol, it is a natural ingredient cannabis plant (also known as marijuana) and hemp plants. cannabinoid-rich oil is extracted from the stalks, stems, and flowers. CBD oil is available in form of capsules, oil bases for vaporizers, tinctures, food items, and beauty products such as bath bombs or lotions. CBD oil and other CBD products are used to treatment of chronic pain, inflammation, migraines, epilepsy, autoimmune diseases, depression, and anxiety.
Centre for drug evaluation and research (CDER) under Food and Drug administration (FDA) is responsible for the evaluation of safety, efficacy and quality of the drugs containing cannabis or cannabis derived products.
CBD Oil Types
Based on product CBD oil is segmented into Marijuana-derived CBD Oil and Hemp-derived oil.
CBD oil Mode of Action
CBD oil is prepared by extracting it from cannabis plant and diluting it with carrier oils, such as coconut oil or hemp seed oil. Cannabinoids is one of the compounds found in cannabis plant, which bind to cannabinoid receptor, which are cell membrane receptors and members of G protein coupled receptors, activated by endocannabinoids, plant cannabinoids and synthetic cannabinoids which are major ligand groups.
CBD oil Applications
CBD oil has shown effectiveness in treatment of some mental disorders, giving natural treatment approach, CBD properties may help to reduce symptoms of depression, anxiety and psychrosis. One of the research study is claiming its effectiveness in reducing PTSD related symptoms including nightmares in adults, may also alleviate certain cancer related symptoms and side effects of cancer treatment like nausea, vomiting, pain.
CBD is helpful in reducing chronic pain, targeting to endocannabinoid receptor activity, helping to reduce inflammation and interact with neurotransmitters, some research preclinical evidence suggests its effectiveness for rheumatic diseases like fibromyalgia.
Research indicates that CBD may be helpful for people with high blood pressure, as it can influence the contractions of the heart muscle and help to widen blood vessels.
It has applications in the cosmetics industry in order to treat skin problems like acne pain-relieving properties, and as it regulates the endocannabinoid system (ECS) activity and interacts with neurotransmitter, it is an ideal ingredient for anti-depression and anxiety drugs
CBD Oil Product development
FDA has issued guidance, prepared by the Office of Pharmaceutical Quality in the Centre for Drug Evaluation and Research (CDER) at the Food and Drug Administration (FDA), providing recommendatory guidelines for sponsors developing such cannabis and cannabis-derived compounds for use in human drugs for clinical research.
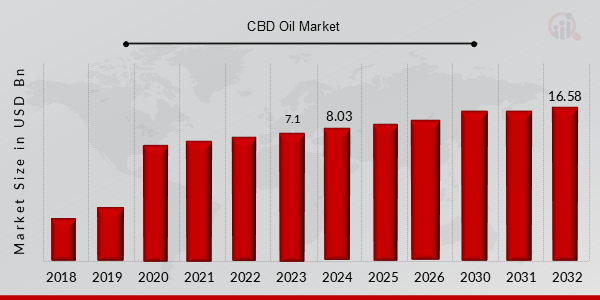
CBD Oil (Cannabidiol Oil) Market Valued at USD 7.1 billion in 2023, projected to grow from USD 8.0 billion in 2024 to USD 16.58 billion by 2032, exhibiting a CAGR of 13.20%. during the forecast period (2024 - 2032). Increased adoption of CBD (Cannabidiol) in medical applications, pet care, and health and fitness, and legalization of using CBD in products are the key market drivers enhancing the growth of market.
Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
CBD Oil Regulatory Landscape:
There are several key regulatory agencies who oversee the approval and monitoring of CBD Oil to ensure their safety, efficacy, and quality.
| Regulatory agencies | Regulatory Ministry |
| Federal Food and Drug Administration | United States: Department of Health and Human Services (HHS) |
| The Medicines and Healthcare products Regulatory Agency | United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
| Central Drug Standard Control Organization | India: The Ministry of Health and Family Welfare |
| Health Canada | Canada: The Ministry of Health |
| Pharmaceuticals and Medical Devices Agency (PMDA) | Japan: Ministry of Health, Labour and Welfare. |
| National Medical Products Administration (NMPA) | China: The Ministry of Health |
| Health Sciences Authority | Singapore: The Ministry of Health |
| European Medicine Agency | European union |
| Therapeutic Goods Administration (TGA) | Commonwealth of Australia |
CBD Oil Guidelines:
Eligibility: eligibility for CBD oil treatment varies globally due to different legal frameworks, medical guidelines and product standards. It is mostly administered to the patients suffering with Epilepsy, chronic pain, anxiety and depression, neurological disorders. It is possibly safe to take in appropriate doses, up to 200 mg daily have been used safely for 13 weeks, with guidance of healthcare provider. CBD treatment can be unsafe to pregnant or breast-feeding women, as CBD products can be contaminated with other ingredients which can cause harm to fetus or infant. Some research suggest increased use of CBD may result in pressure on eye, and cause glaucoma in some patients, some other research suggest high dose of CBD can make muscle and tremors worse in some patients with Parkinson’s disease.
CBD Oil Classification of the Product:
CBD Oil Regulatory Process Overview, By Country:
FDA has issued a recommendatory guidance on cannabis and cannabis derived compounds: quality considerations for clinical research, for ensuring safety, efficacy and quality of the drugs containing cannabinoids.
For these drugs to be legally marketed in interstate commerce, drugs which are not biological products must generally get premarket approval by FDA through New Drug Application (NDA) OR abbreviated new drug application (ANDA) process, meeting the requirements in the FD&C Act for market approval.
2018 farm bill has legalised hemp (≤ 0.3% delta-9 Tetrahydrocannabinol (THC) by dry weight), hemp is no longer a controlled substance under Federal law, but cannabis above this threshold remains a schedule 1 controlled substance under controlled substance act (CSA).
Lead federal agency for regulating the controlled substances is Drug Enforcement Administration (DEA). Growing ad manufacturing activities related to cannabis for use of investigational drug for research must comply with CSA and DEA requirements.
FDA’s has Cannabis Product Committee (CPC) for development and implementation of cross-Agency strategy and policy for the regulation of cannabis products.
Sources of cannabis
National Institute on Drug Abuse (NIDA) Drug Supply Program (DSP) is providing domestic and federally legal source of cannabis for clinical research for many years. Cannabis for DSP is grown under contract by university of Mississippi at national centre for natural products research.
Cannabis sources with no more than 0.3 percent delta-9 THC on a dry weight basis and with over 0.3 percent delta-9 THC on a dry weight basis may be used for clinical research if deemed to be of adequate quality by FDA when reviewed as part of an IND.
Quality and manufacturing considerations
As a part of IND for the drug, Sponsors should ensure consistent manufacturing of high-quality cannabis derived drugs. Each clinical phase requires increasing levels of identity, quality, purity, and potency data.
FDA guidance documents available
-
Phase 1 INDs – follow FDAs CGMP for phase 1 investigational drugs (2008)
-
Phase 2 and 3 INDs – adhere to INDs for phase 2 and phase 3 studies- CMC information (2003).
Drug developers must characterise cannabis materials ensuring batch to batch consistency and compliance with current good manufacturing practices (CGMP).
Analytical testing should include THC content, impurities, microbiological contaminants and residual pesticides.
Considerations for control status under CSA
-
If drug formulation exceeds 0.3% delta-9 THC at any stage, it becomes a controlled substance under DEA regulations.
-
Developers should calculate THC content on dry weight basis to determine control status.
-
FDA may require abuse potential assessments for cannabis derived drug products.
CBD Oil regulatory challenges
Uncertainty in regulatory framework is one of the most major challenges for CBD industry. Quality control issues due to absence of industry wide standards, leading to variations in product quality, instances of contamination with pesticides, heavy metals and even THC, marks need of strict quality control measures. Labelling issues, like discrepancies between CBD content claimed on labels of product and the content present in product, these inaccuracies complicate regulatory efforts for ensuring safety, efficacy and quality.
The legal status of CBD products varies across the globe, even within countries, for instance, in United States, there can be conflict between federal and state laws. Inconsistency causes issues in development of uniform standards and complicates enforcement efforts. Certain jurisdictions still classify CBD as a controlled substance, akin to its psychoactive counterpart, THC, this can cause regulatory hurdles in production, distribution, and research related to CBD products.
Risk in development of CBD Oil
Market Saturation: The rapid growth of the CBD market has led to saturation, with countless products taking for consumer attention. This increase makes it difficult for regulators for monitoring and evaluating, every product for compliance with potential standards.
CBD Oil Competitive Landscape Dashboard:
Companies With Marketed CBD Oil:
-
Jazz Pharmaceutical plc.
-
Elixinol Wellness Limited
-
ENDOCA
-
Medical Marijuana Inc.
-
Nuleaf Naturals LLC
-
Isodiol International Inc.
-
Pharmahemp doo
-
Folium Biosciences
-
Cannoid LLC
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”