
Regulatory Landscape - Overview
3D bioprinting Regulatory Landscape: Product Overview
3D bioprinting is a form of additive manufacturing, involving layer by layer deposition of bioinks, which is the material composed of living cells or biomolecules and biocompatible polymers to fabricate complex, functional biological structures such as tissues and organs.
This technology mimics natural architecture of human tissues and is primarily used in regenerative medicine, tissue engineering, and pharmaceutical research.
3D bioprinting types
- Inkjet Bioprinting: uses thermal or piezoelectric actuators to eject droplets of bioink onto hydrogel substrate or culture dish under computer control.
- Extrusion based Bioprinting: involves continuous dispensing of bioink through nozzle using pneumatic or mechanical pressure.
- Laser Assisted Bioprinting: Uses laser pulse to transfer bioink from a donor slide to receiving substrate.
- Stereolithography based Bioprinting: uses light, mostly UV to polymerise photosensitive bioinks in a layer wise manner.
3D Bioprinting Applications
The 3D Bioprinting is rapidly evolving within the areas such as tissue engineering, drug testing, organ printing, and cancer research. tissue Engineering is particularly significant, as it is dedicated to developing biological substitutes that can restore, maintain, or improve tissue function.
Drug Testing benefits greatly from this technology by allowing for more accurate human tissue models, which improve testing efficiency and reduce lead times in drug development. Organ Printing showcases the ability to create functional human organs, addressing critical shortages of organ donors globally.
Cancer Research utilizes bioprinting to create realistic tumor models, enabling researchers to conduct advanced studies on treatment efficacy. This segment is driven by the increasing need for personalized medicine and innovative approaches to healthcare, emphasizing the relevance of these applications in improving patient outcomes and advancing medical technologies.
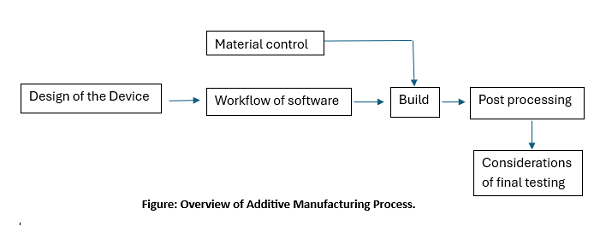
3D Bioprinting Product Development steps.
- Making Design of the process is the very first step in additive manufacturing process, which includes standard design with discrete pre-specified sizes and models or patient-matched device (PMD), which is designed from own medical images of the patients.
- Thence the device design is converted to digital file and initiates software workflow phase, which involves further processing of the file and preparing it for the printing.
- Then the optimization of the printing parameters is done, and the machine ready file is produced from the build file.
- Along with these steps, establishment of the material controls for materials utilised in device printing is also carried out.
- Once the printing is complete, built device or any built component is taken for post processing step involving cleaning, annealing, post-printing machining, sterilization, packing and Labeling.
- Upon completion of post-processing step, the final finished device is ready for testing and characterization, quality system must be applied across all of these processes.
3D Bioprinting Market Size Overview
As per MRFR analysis, the 3D Bioprinting Market Size was estimated at 1.14 (USD Billion) in 2024.The 3D Bioprinting Market Industry is expected to grow from 1.29(USD Billion) in 205 to 5 (USD Billion) by 2035. The 3D Bioprinting Market CAGR (growth rate) is expected to be around 13.08% during the forecast period (2025 - 2035).
3D Bioprinting Regulatory Landscape:
There are several key regulatory agencies who oversee the approval and monitoring of 3D Bioprinting to ensure their safety, efficacy, and quality.
|
Regulatory agencies |
Regulatory Ministry |
|
Federal Food and Drug Administration |
United States: Department of Health and Human Services (HHS) |
|
The Medicines and Healthcare products Regulatory Agency |
United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
|
Central Drug Standard Control Organization |
India: The Ministry of Health and Family Welfare |
|
South African Health Products Regulatory Authority (SAHPRA) |
National Department of Health. |
|
Pharmaceuticals and Medical Devices Agency (PMDA) |
Japan: Ministry of Health, Labour and Welfare. |
|
National Medical Products Administration (NMPA) |
China: The Ministry of Health |
|
Health Sciences Authority |
Singapore: The Ministry of Health |
|
European Medicine Agency |
European union |
|
Brazilian Health Regulatory Agency (Anvisa) |
Ministry of Health, part of the Brazilian National Health System (SUS) |
3D Bioprinting Guidelines:
Patients who require reconstruction of different types of body tissues is possible due to 3D bioprinting, providing artificial organs with natural characters for transplants and surgeries, it is a cost effective approach in healthcare system, as 3D printed organs are less costly than the natural organs, and organ shortage is major problem in healthcare due to which healthcare professionals are unsuccessful to save lives of many patients with organ failure issue, In US, more than 10,000 people are waiting for their organ transplant, Entire globe is running short of organs. Organ donations are not enough to meet the demand, 3D bioprinting can become the best treatment option who can give customized artificial organs or tissues or customization in regenerative medicine, even overcoming issues or donor rejection and saving the lives of many patients.
As animal testing has reduced due to 3D bioprinting, number of animals killed annually, due to use in clinical studies and trials have also reduced. Indirectly, this technology is protecting our ecosystem.
3D Bioprinting Classification of the Product:
3D Bioprinting Regulatory Process Overview, By Country:
3D bioprinting fall under the category of additive manufacturing in medical devices. These devices must comply with the existing regulations for medical devices and sometimes depending on their composition they may fall under combination products oversight involving drugs, biologics or tissues.
FDA has issued guidance document “Technical considerations for additive manufactured devices” to provide recommendatory guidelines for device design, manufacturing, and testing considerations when developing 3D printed devices. This document is divided into two sections:
Device Design and manufacturing Considerations
Patient-matched devices (PMD) can be produced using various methods, including additive manufacturing (AM) and traditional techniques. AM is particularly suitable for manufacturing PMD, and this guidance provide relevant considerations which include:
- PMD can be based on standard-sized templates matched to a patient's anatomy or produced within a defined design/performance envelope.
- The performance envelope includes minimum and maximum dimensions, mechanical performance limits, and other clinically relevant factors.
- Patient-matching can be achieved through scaling using anatomical references or full anatomical features from patient imaging.
- PMD are not considered "customized" devices under the FD&C Act unless they meet specific criteria of section 520(b).
- Most PMD will follow existing regulatory pathways for their device type. PMD designs may be modified by clinical staff, manufacturers, or third parties based on clinical inputs from measurements, assessments, or imaging.
- It's important to identify clinically relevant design parameters, their pre-determined ranges, and which parameters can be modified for patient-matching.
- Proper management of personally identifiable information (PII) and protected health information (PHI) is most important in clinical applications.
Device testing considerations
This section of the guidance describes the type of information that should be provided in premarket submissions for a 3D printed device made using AM.
It should include all the information related to the product like, device description, data gathered through mechanical testing which include material property testing (like modulus, strength. Viscoelasticity, fatigue), dimensional measurements, material characterization which include the data related to all the materials involved in manufacturing of device, for the review process certificate of analysis and/or materials safety data sheets (MSDS) and chemical abstract service number, of each chemical component should be provided, then data related physical properties of the material like inter layer bonding which is unique to the AM, determining ultimate structural integrity of the final finished device must also be included, biocompatibility testing data and labelling.
Key regulatory submissions that may be required for such devices include:
- Premarket notification 510 (k): It is a premarket submission which ensure that the device safe and effective, and it is substantially equivalent to any legally marketed device.
- Premarket Approval (PMA) Premarket approval (PMA) is the FDA process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices. Class III devices are those that support or sustain human life.
- De Novo Classification Requests The De Novo classification process by the FDA is a regulatory pathway for medical devices that do not have a legally marketed predicate device. It allows for the classification of devices into Class I or Class II based on their risk level.
- Investigational Device Exemption IDE is to allow use of investigational device in a clinical study for collecting safety and effectiveness data, which is further used to support the Premarket Approval.
3D Bioprinting updates
November 2022, Avay Biosciences, a Chennai-based startup, has developed an advanced Bio 3D printer named Mito Plus that can print human tissues. This bioprinter uses "bioinks" to create synthetic living tissues, such as skin, which can be used for pharmaceutical drug discovery, drug testing, cosmetology, and cancer biology. The first prototype was installed at the Indian Institute of Science in Bengaluru. This innovation represents a significant step forward in bioprinting technology in India.
April 2019, Researchers in Israel have achieved a success by 3D printing a personalized heart using a donor's tissue. This heart includes cells, blood vessels, ventricles, and chambers, making it a significant advancement in bioprinting technology. The heart was created using reprogrammed cells and biological materials from the donor, which could potentially reduce the risk of rejection by the patient's immune system. Although the printed heart is currently the size of a rabbit's heart and not yet fully functional, this development marks a promising step towards future applications in organ transplants.
3D Bioprinting Regulatory Challenges and possible risk in development:
Determining the appropriate regulatory classification for 3D bioprinted products can be complex due to their combining elements of medical devices, biologics, and drugs.
Evaluating the risks associated with 3D bioprinted products is challenging because of the variability in materials, processes, and the inclusion of living cells. screening of prospective donors, and the storage, transportation of the biologic is one of the major challenge. autologous cell or tissue therapies screening is more challenging, where clinicians perform the extraction, manipulation and reimplantation steps.
Even wide diversity of cell therapy products involved in 3D bioprinting requires its own set of preparation processes and mechanisms of delivery, often challenging to cover under one regulatory policy.
Another challenge is lack of harmonization across different countries, creating challenge for the manufacturers to develop product which will be accepted in most countries. There is a lack of standardized protocols and guidelines for the production and quality control of 3D bioprinted products, making it difficult to ensure consistency and safety.
Ensuring the quality and reproducibility of 3D bioprinted products is difficult due to the complexity of the manufacturing process. The incorporation of living cells and the complexity of the manufacturing process present additional technical challenges that need to be addressed in the regulatory framework.
3D Bioprinting Competitive Landscape Dashboard:
Companies With Marketed 3D Bioprinting:
- GeSim
- Aspect Biosystems
- Organovo
- TeVido BioDevices
- TissUse
- Xtent
- EnvisionTEC
- CELLINK
- Stratasys
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”