
Regulatory Landscape - Overview
Nanobiomedicine Regulatory Landscape: Product Overview
Nanomedicine is known to be one of the applications of nanotechnology that is utilized in the diagnosis, treatment, monitoring, and control of biological systems. Nanomedicine utilizes nanoscale manipulation of materials to augment medicine delivery. Therefore, nanomedicine has enabled the treatment of several diseases. Nanomedicine is in lucrative stages as various products are in the development phase.
Centre for drugs evaluation research (CDER) and centre for biologics evaluation and research (CBER) of Food and Drug Administration FDA is looking after all the activities related to the regulation of the therapeutics involving the use of nanomaterials, for ensuring the safety, efficacy and quality of the products.
Nano biomedicine Types
Based on modality, the nanomedicine has been segmented into treatments and diagnostics, and depending on application, it is classified into drug delivery, diagnostic imaging, vaccines, regenerative medicine, and implants.
Nanomedicines can be broadly divided into various categories based on:
| Morphology | Structure | Composition |
| Nanoparticles Micelles Nanofibers | Nano capsules Nanospheres Nanocrystals | Lipid based nanoparticles Polymeric nanoparticles Protein nanoparticles Inorganic nanoparticles Carbon nanoparticles |
Nano biomedicine Applications
In the treatments, nanomedicine presents novel methods for regenerative medicine, targeted therapy, and drug delivery. Drugs are delivered to diseased cells directly using nanoparticles, reducing adverse effects and increasing treatment efficacy. Targeting tumour cells without damaging healthy tissue makes this precision treatment method especially important in oncology, where it can help patients with cancer get better outcomes.
In diagnostics, nanomedicine makes it feasible to develop highly specific and sensitive diagnostic instruments. Early identification of conditions including cancer, heart disease, and neurological illnesses is made possible by the improved detection of biomarkers at very low concentrations made possible by nanoparticles and nano sensors. Improved patient outcomes and prompt care depend on this early discovery. Additionally, deeper insights into disease pathology are provided by nanomedicine-based imaging modalities, such as nanoparticle-enhanced MRI and CT scans, which help with more precise diagnosis and personalized therapy planning.
The use of nanoparticles to enhance bioavailability, stability, and targeting of therapeutic agents is a significant use of drug delivery systems. This precision, in particular in cancer treatment, minimizes side effects and improves the effectiveness of therapy.
In diagnostic imaging, nanomedicine has developed advanced diagnostic imaging techniques that are more accurate and sensitive than conventional techniques. Nanoparticles are a contrast agent in imaging modalities such as MRI, CT scan, and PET scans that improve the clarity and detail of tissue and organs. This will improve the detection and accurate diagnosis of diseases, thus providing timely and efficient treatment. Additionally, the development of vaccines has also been transformed by nanomedicine, with nanoparticles being used as adjuvants in order to stimulate immune responses or delivery vehicles for ensuring a stable and effective presentation of antigens. In the case of the rapid development and high efficacy of nanoparticle vaccines, this has a significant impact on infectious diseases such as COVID-19, for which vaccines based on nanoparticles have demonstrated quick development and excellent efficacy.
Regenerative medicine, in which damaged tissues and organs are repaired and regenerated with the use of nanomaterials. The rising prevalence of degenerative illnesses, developments in tissue engineering and stem cell research, and rising funding for regenerative medicine research are the main factors driving this market. Furthermore, nanotechnology-enhanced implants provide better functionality, longevity, and biocompatibility. Implants are coated with nano-coatings or nanocomposites to lower the risk of infection, improve the device's integration with biological tissues, and increase its longevity.
Nano biomedicine Product development
Centre for drug evaluation and research (CDER) nanotechnology working group of FDAs along with Centre for biologics evaluation and research (CBER) has issued guidance for regulation of drug containing nanomaterials.
Documents give guidance for developing human drug products, in which nanomaterial is present in finished dosage form, focusing on considerations relevant to FDAs regulation of these drug products under the Federal Food, Drug, & Cosmetic Act (FD&C Act) and Public Health Service Act (PHS Act), includes recommendatory guidelines for sponsors and applicants of investigational, premarket, post market submission of the products, furthermore, it includes recommendations regarding the National Environmental Policy Act (NEPA), as relevant to potential FDA regulatory decisions on these drug products.
FDA has issued regulatory guidance for developing drugs with nanomaterials, which gives recommendations to industry related to:
-
Risk based evaluation recommendation by FDA
-
Quality evaluation recommendations by FDA
-
Manufacturing and control
-
Nonclinical studies
-
Clinical development
-
Environmental impact considerations
-
Post market surveillance and regulatory compliance
list of some of the approved Nano biomedicine by FDA
| Nano biomedicine approved | Manufacturer | Indication | Approval date |
| Pfizer BioNTech Vaccine | Pfizer Pharmaceuticals | mRNA vaccine | 2020 |
| Moderna COVID 19 vaccine | Moderna TX Inc. | mRNA Vaccine | 2020 |
| Onpattro | Alnylam | Hereditary transthyretin mediated amyloidosis | 2018 |
| Vyxeos | Jazz pharmaceuticals | Acute myeloid leukaemia | 2018 |
Nano biomedicine Market Size Overview:
As per MRFR analysis, the Nano biomedicine Market Size was estimated at 235.43 (USD Billion) in 2022. The Nano biomedicine Market Industry is expected to grow from 252.97 (USD Billion) in 2023 to 819.84 (USD Billion) till 2032, at a CAGR (growth rate) is expected to be around 12.48% during the forecast period (2022-2032). Iincreasing incidences of various diseases across the globe, agreements associated with nanomedicine products developments are the key market drivers enhancing the growth of the market.
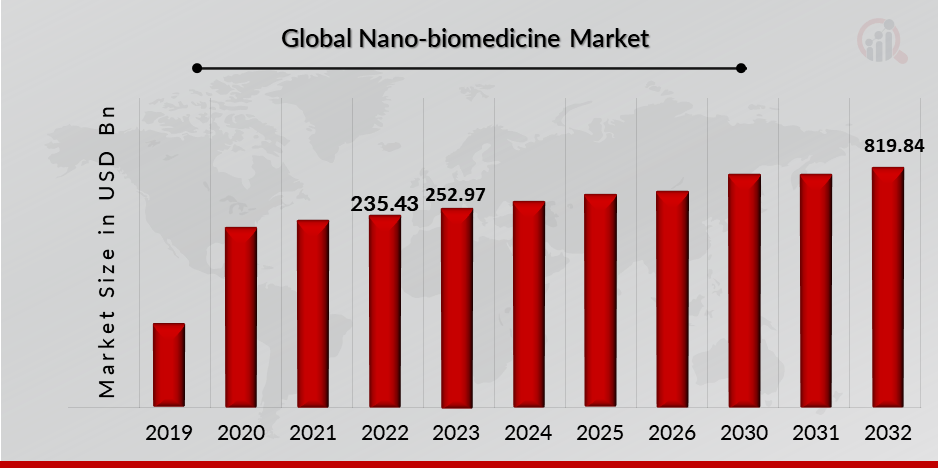
Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
Nano biomedicine Regulatory Landscape:
There are several key regulatory agencies who oversee the approval and monitoring of Nano biomedicine to ensure their safety, efficacy, and quality.
| Regulatory agencies | Regulatory Ministry |
| Federal Food and Drug Administration | United States: Department of Health and Human Services (HHS) |
| The Medicines and Healthcare products Regulatory Agency | United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
| Central Drug Standard Control Organization | India: The Ministry of Health and Family Welfare |
| Health Canada | Canada: The Ministry of Health |
| Pharmaceuticals and Medical Devices Agency (PMDA) | Japan: Ministry of Health, Labour and Welfare. |
| National Medical Products Administration (NMPA) | China: The Ministry of Health |
| Health Sciences Authority | Singapore: The Ministry of Health |
| European Medicine Agency | European union |
| Therapeutic Goods Administration (TGA) | Commonwealth of Australia |
Nano biomedicine Guidelines:
Eligibility: Nano biomedicine is used in various medical fields, it can be administered for the treatment of cancer, where patients are given targeted therapy, with less side effects. It can be used by diabetic patients, using nanotechnology-based glucose sensors.
Nano biomedicine Classification of the Product:
Nano biomedicine Regulatory Process Overview, By Country:
The FDA Nanotechnology Task Force (Task Force), formed in August 2006, was charged with determining regulatory approaches that encourage the continued development of innovative, safe, and effective FDA-regulated products that use nanotechnology or nanomaterials.
Risk based evaluation recommendations by FDA
According to FDA guidance appropriate regulatory frameworks for potential risk evaluation of nanomaterial containing drug products should ensure following things:
-
Adequate characterisation of nanomaterials structure and function
-
Adequate knowledge of intension of nanomaterials use and application and understanding of relation between nanomaterials attribute with product quality, safety and efficacy.
-
Complexity in structure of material
-
Understanding of mechanism of Physiochemical properties of the material impacting on its biological effects (effect of pharmacokinetic parameters on particle size)
-
Based on materials physiochemical properties understanding of mechanism of in vivo release.
-
Understanding of physical and chemical stability of nanomaterials.
-
Nanotechnology maturity in manufacturing and analytical methods.
-
Impact of changes in manufacturing, including in process controls and control strategies robustness on critical quality attributes (CQA) of drug product.
-
Materials physical state upon administration, administration route
-
Information related to dissolution, distribution, biodegradation
-
Predictions based on animal study and physicochemical parameters.
Quality evaluation recommendations by FDA
-
Premarket application should include details of nanomaterial used in drug product, describing the product life cycle, structure, charge, composition, functionality, and size.
-
For consistent quality of drug products, sufficient description of nanomaterial in ANDA, NDA, BLA should be submitted.
-
Determination of nanomaterial’s CQA -critical quality attributes, in terms of its function and potential impact on product performance.
-
Narrative description and its complementary diagram should be included, providing only ingredients list is not sufficient for explaining resulting structure of the nanomaterial after assembly, formulation, and/or processing.
-
Apply physicochemical characterisation methods for assessing structure of material and to ensure consistency, factors like method suitability, complementary methods, sampling, sample preparation should be considered while using specific characterisation methods.
-
Developing dissolution and in vitro drug release method to evaluate release and performance of drug product, premarket application should include all the information about proposed dissolution/in vitro release test and the developmental parameters (selection of equipment/apparatus, media, agitation/rotation speed, pH, sink conditions, surfactant type and concentration).
-
Ensuring compliance with current good manufacturing practices (CGMP) set forth in section 501(a)(2)(B) of the FD&C Act.
-
Establishing robust process control for maintaining batch consistency and preventing contamination.
-
In process monitoring of nanomaterial implementation throughout the production cycle.
-
Evaluation of manufacturing changes impact on performance and quality of drug.
-
Conducting adsorption, distribution, metabolism, and excretion (ADME) study, for assessing biodistribution and clearance of nanomaterials.
-
Study of potential toxicological effects, including accumulation in organs and altered biological interactions.
-
consideration of risk associated with specific route of administration
-
Conducting toxicology study for comparative analysis of nanomaterial contain drugs with conventional formulations.
Submitting applications through relevant regulatory pathways
-
505 (b) (2) pathway, this pathway is ideal for modified or improved versions of existing innovator drugs, leading to the creation of a distinct drug product with its own exclusivity rights. The process incorporates pre-existing data and new findings to facilitate a more efficient approval process.
-
505 (j) pathway for generic drugs having nanomaterials, an applicant may seek approval of a generic product that references a drug product containing nanomaterials by submitting an ANDA under section 505(j) of the FD&C Act.
-
351 (k) pathway for biosimilars, the development of a biosimilar to a biological reference product containing nanomaterials should generally follow current guidance on biosimilars.
Conducting bioanalytical studies parent drug and major active metabolites, if possible, should be measured in the appropriate biologic matrices after administration of products containing nanomaterials. In vitro test with human biomaterials, for evaluation of drug stability and bioavailability, plasma protein binding, in vitro clearance and metabolism. And assessing immunogenicity risks.
Environmental impact considerations
Assessing environmental risks under national environmental policy act (NEPA), conducting environment impact assessments for nanoparticles that may pose ecological risks. Involve use of case-by-case evaluation.
Post market surveillance and regulatory compliance
Monitoring safety and efficacy of the product event after approval.
Nano biomedicine regulatory challenges
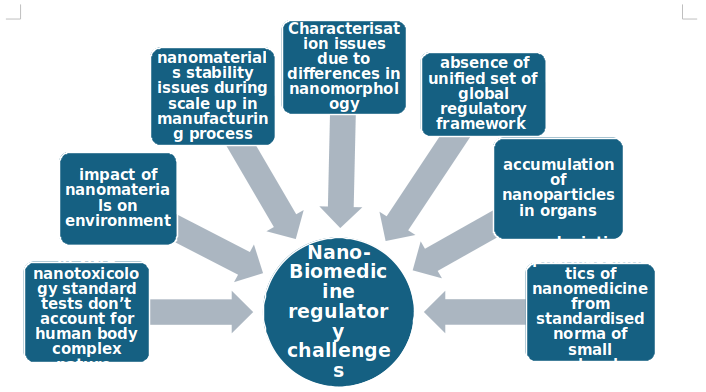
Source: MRFR analyses
Risk in development of Nanomedicine
High Manufacturing Cost of Nanomedicine
The high cost associated with the research, development, and production of nanomedicine products limits their accessibility and affordability. Moreover, there is also a high developmental cost of nanomedicine due to unspecified standards and regulations and unknown biological interactions, effects, and toxicities. Manufacturing nanoscale drug delivery systems and nanoparticles involves complex and specialized processes, often requiring advanced equipment, precise control over materials, and stringent quality assurance measures. These sophisticated manufacturing techniques result in elevated production costs compared to traditional pharmaceutical manufacturing methods. Additionally, the synthesis and formulation of nanomedicine products may necessitate expensive raw materials and specialized facilities, further adding to the overall manufacturing expenses. As a result, the high manufacturing costs pose a barrier to entry for smaller companies and startups in the nanomedicine sector, limiting innovation and competition. Thus, the above-mentioned reasons, which add up to the high development cost of nanomedicine, are restraining the global nanomedicine market.
Nano Biomedicine Competitive Landscape Dashboard:
Companies With Marketed Nano Biomedicine:
-
Sanofi
-
Johnson & Johnson services, Inc
-
Parvus Therapeutics Inc
-
Nanobiotix
-
Ascandia Pharmaceuticals
-
Bristol Myers Squibb Company
-
Jazz Pharmaceuticals
-
Cytimmune Sciences, Inc
-
Nanospectra Biosciences
-
Pfizer Inc
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”