Regulatory Support for Gene Therapies
Regulatory bodies are increasingly providing support for the development and approval of gene therapies, which serves as a vital driver for the Adeno-associated Virus (AAV) Vector-based Gene Therapy Market. Initiatives aimed at expediting the review process for gene therapies are becoming more common, with agencies implementing frameworks that facilitate faster access to innovative treatments. This regulatory environment encourages companies to invest in AAV vector technologies, as the path to market becomes clearer and more efficient. The approval of several AAV-based therapies in recent years underscores this trend, suggesting a favorable landscape for future developments in the market.
Rising Prevalence of Genetic Disorders
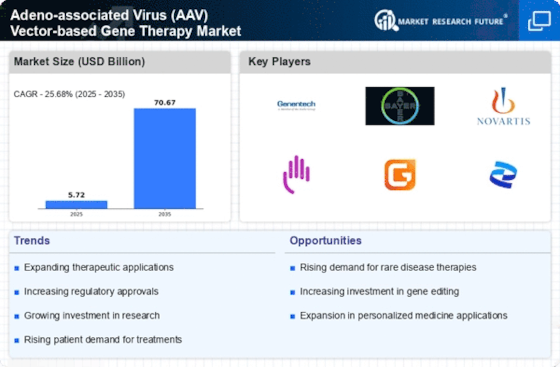
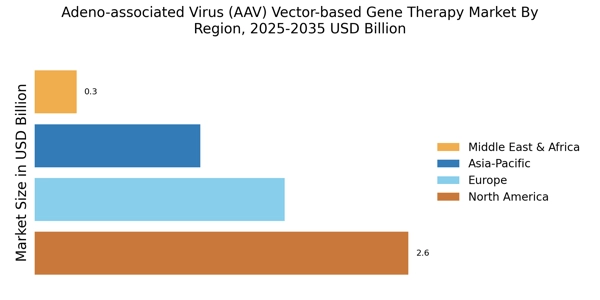
The increasing incidence of genetic disorders is a primary driver for the Adeno-associated Virus (AAV) Vector-based Gene Therapy Market. As more individuals are diagnosed with conditions such as hemophilia, muscular dystrophy, and cystic fibrosis, the demand for effective gene therapies rises. According to recent estimates, genetic disorders affect approximately 1 in 300 births, highlighting a substantial patient population in need of innovative treatments. This growing prevalence necessitates the development of targeted therapies, which AAV vectors are well-suited to provide. The ability of AAV vectors to deliver therapeutic genes effectively into target cells positions them as a promising solution in addressing these disorders, thereby propelling market growth.
Growing Investment in Biopharmaceuticals
The surge in investment within the biopharmaceutical sector is a crucial driver for the Adeno-associated Virus (AAV) Vector-based Gene Therapy Market. As pharmaceutical companies and venture capitalists increasingly allocate funds towards gene therapy research, the development of AAV-based therapies is gaining momentum. Reports indicate that the biopharmaceutical market is expected to grow significantly, with projections suggesting it could surpass hundreds of billions in value. This influx of capital not only accelerates research and development but also enhances collaboration between academic institutions and industry players, fostering innovation in AAV vector technologies. Such dynamics are likely to lead to the introduction of new therapies, further stimulating market growth.
Advancements in Gene Editing Technologies
Technological advancements in gene editing are significantly influencing the Adeno-associated Virus (AAV) Vector-based Gene Therapy Market. Innovations such as CRISPR and TALENs have enhanced the precision and efficiency of gene modification, creating a synergistic effect with AAV vectors. These technologies enable researchers to develop more effective therapies that can target specific genetic mutations. The market for gene editing is projected to reach substantial figures, with estimates suggesting it could exceed several billion dollars in the coming years. This intersection of gene editing and AAV vector technology is likely to foster the development of novel therapies, thereby expanding the market and attracting investment.
Increased Awareness and Acceptance of Gene Therapies
The growing awareness and acceptance of gene therapies among healthcare professionals and patients are pivotal drivers for the Adeno-associated Virus (AAV) Vector-based Gene Therapy Market. As educational initiatives and successful case studies emerge, the perception of gene therapies is shifting positively. Surveys indicate that a significant percentage of healthcare providers are now more inclined to recommend gene therapies, reflecting a broader acceptance of these innovative treatments. This shift is likely to enhance patient demand for AAV-based therapies, as individuals become more informed about their options. Consequently, this increased acceptance may lead to higher adoption rates, further propelling market growth.