Expansion of Clinical Trials
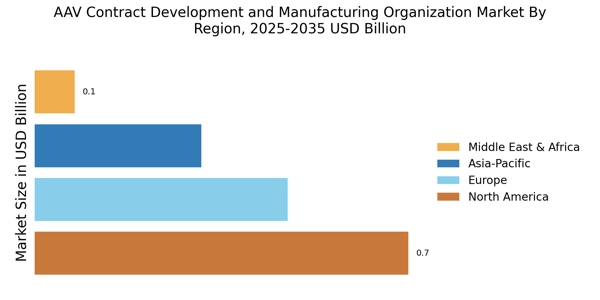
The AAV Contract Development and Manufacturing Organization Market is witnessing an expansion in clinical trials, which is a critical driver of market growth. As more companies initiate clinical trials for AAV-based therapies, the demand for specialized manufacturing services increases. In 2025, it is estimated that over 200 clinical trials involving AAV vectors are underway, reflecting a growing interest in their therapeutic applications. This expansion necessitates the collaboration with AAV contract development organizations that can provide the necessary infrastructure and expertise to support these trials. The ability to scale production in response to trial demands is vital, and AAV contract manufacturers are well-positioned to meet these needs. Thus, the expansion of clinical trials serves as a pivotal factor propelling the AAV Contract Development and Manufacturing Organization Market.
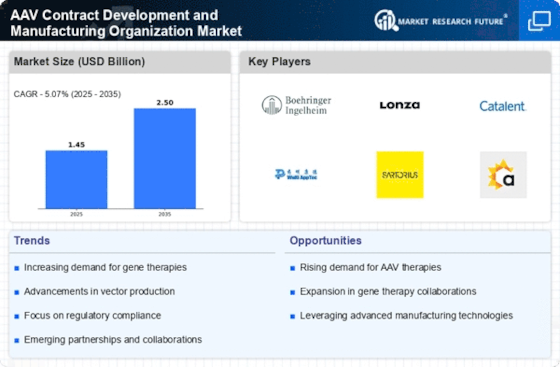
Rising Investment in Gene Therapies
The AAV Contract Development and Manufacturing Organization Market is experiencing a surge in investment as gene therapies gain traction. This increase in funding is driven by the growing recognition of the potential of gene therapies to treat previously untreatable conditions. In 2025, the market for gene therapies is projected to reach approximately 10 billion USD, indicating a robust growth trajectory. As pharmaceutical companies and biotech firms seek to capitalize on this trend, they are increasingly outsourcing their manufacturing needs to AAV contract development organizations. This outsourcing allows for greater flexibility and access to specialized expertise, which is essential for the successful development and commercialization of gene therapies. Consequently, the rising investment in gene therapies is a significant driver for the AAV Contract Development and Manufacturing Organization Market.
Increasing Prevalence of Genetic Disorders
The increasing prevalence of genetic disorders is a significant driver for the AAV Contract Development and Manufacturing Organization Market. As the global population ages and genetic conditions become more common, the demand for effective therapies rises. In 2025, it is estimated that over 400 million individuals worldwide are affected by genetic disorders, creating a pressing need for innovative treatment solutions. AAV vectors have emerged as a promising platform for delivering gene therapies aimed at these conditions. Consequently, pharmaceutical companies are investing in AAV-based therapies, leading to a higher demand for contract manufacturing services. AAV contract development organizations play a crucial role in supporting the development and production of these therapies, thereby driving growth in the market. The increasing prevalence of genetic disorders underscores the importance of the AAV Contract Development and Manufacturing Organization Market.
Regulatory Support for Gene Therapy Development
Regulatory support for gene therapy development is emerging as a vital driver for the AAV Contract Development and Manufacturing Organization Market. Regulatory agencies are increasingly recognizing the potential of gene therapies and are streamlining approval processes to facilitate their development. In 2025, several countries have implemented expedited pathways for gene therapy products, which encourages investment and innovation in this field. This supportive regulatory environment not only accelerates the time to market for AAV-based therapies but also enhances the attractiveness of outsourcing manufacturing to specialized AAV contract development organizations. As companies seek to navigate the complex regulatory landscape, they rely on the expertise of these organizations to ensure compliance and successful product launches. Thus, regulatory support for gene therapy development is a crucial factor driving the growth of the AAV Contract Development and Manufacturing Organization Market.
Technological Advancements in Manufacturing Processes
Technological advancements are significantly influencing the AAV Contract Development and Manufacturing Organization Market. Innovations in manufacturing processes, such as improved vector production techniques and enhanced purification methods, are enabling more efficient and cost-effective production of AAV vectors. In 2025, the adoption of these advanced technologies is expected to reduce production costs by up to 30%, making AAV therapies more accessible. Furthermore, these advancements facilitate compliance with stringent regulatory standards, which is crucial for the successful commercialization of gene therapies. As AAV contract development organizations integrate these technologies into their operations, they enhance their competitive edge and attract more clients. Therefore, technological advancements in manufacturing processes are a key driver of growth within the AAV Contract Development and Manufacturing Organization Market.