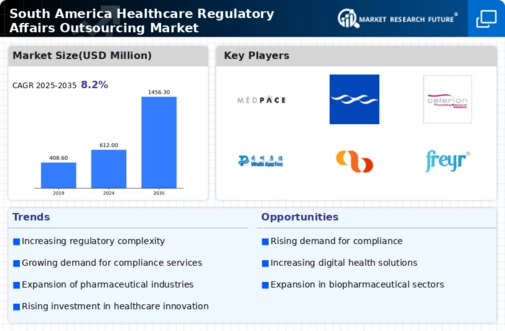
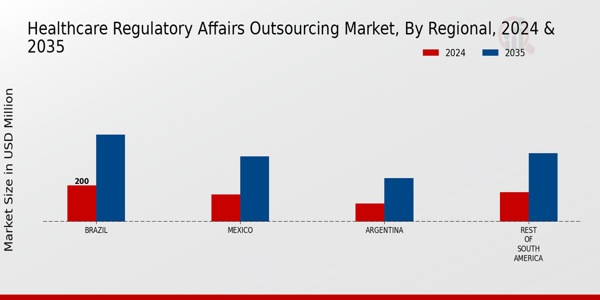
The South America Healthcare Regulatory Affairs Outsourcing Market is a dynamic environment characterized by increasing demand for adherence to regulatory compliance as pharmaceutical and biotechnology companies strive to navigate complex regulations and standards. The region has seen significant growth, driven by the expansion of the healthcare sector, investment in research and development, and the rising need for skilled professionals who understand local regulatory landscapes.
The competitive landscape features a mix of local and international players, each vying to provide specialized services that ensure timely market entry for new medical products while maintaining compliance with national and international regulations. This market presents opportunities for companies that can optimize costs and efficiency while maintaining high-quality service levels across various stages of regulatory affairs, including clinical trial applications, pre-market approvals, and post-market surveillance.
Covance stands out in the South America Healthcare Regulatory Affairs Outsourcing Market due to its strong reputation and comprehensive service portfolio that encompasses both regulatory affairs and contract research organization (CRO) capabilities. The company’s presence in South America is marked by its expertise in facilitating clinical research and ensuring compliance with local regulations, which is essential for successful drug development.
Covance’s strength lies in its ability to leverage its global experience while localizing its services to meet specific regional needs. By collaborating with local healthcare entities and regulatory agencies, Covance effectively enhances its market presence and helps clients streamline their regulatory processes, thus enabling them to bring products to market more efficiently in South America.
Medpace similarly plays a crucial role in the South America Healthcare Regulatory Affairs Outsourcing Market, focusing on providing a full-service clinical research organization experience that includes regulatory expertise. The company’s key offerings involve regulatory consulting, clinical trial management, and strategic guidance on navigating local law compliance.
Medpace’s established presence in South America is characterized by a network of relationships with regulatory authorities, which equips it with the insights necessary for effective market penetration. The firm is recognized for its commitment to quality and efficiency, which reinforces its reputation among clients seeking to expedite their product development timelines. Recently, Medpace has pursued several strategic mergers and acquisitions to enhance its capabilities, thereby expanding its expertise and service offerings in the biotechnology and pharmaceutical sectors within the South American market.
This approach not only strengthens its competitive position but also allows for improved support of clients aiming to comply with the diverse regulatory environments across various countries in the region.



















Leave a Comment