Cost Efficiency and Resource Optimization
In the current economic climate, organizations are seeking ways to optimize resources and reduce operational costs. The healthcare regulatory-affairs-outsourcing market is benefiting from this trend, as outsourcing regulatory functions can lead to significant cost savings. By leveraging external expertise, companies can avoid the expenses associated with maintaining in-house regulatory teams. Reports indicate that outsourcing can reduce operational costs by up to 30%, allowing organizations to allocate resources more effectively. This financial incentive is likely to drive further growth in the healthcare regulatory-affairs-outsourcing market, as businesses aim to enhance their bottom line while ensuring compliance with regulatory standards.
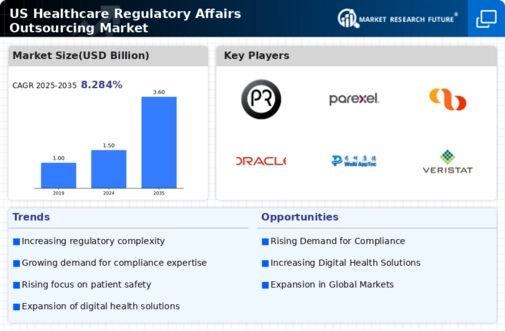
Rising Complexity of Regulatory Frameworks
The healthcare regulatory-affairs-outsourcing market is experiencing a notable increase in complexity due to evolving regulations. Regulatory bodies, such as the FDA, are continuously updating guidelines to ensure patient safety and product efficacy. This complexity necessitates specialized knowledge, prompting companies to outsource regulatory affairs to experts who can navigate these intricate frameworks. As of 2025, the market for regulatory affairs outsourcing is projected to grow at a CAGR of approximately 10%, driven by the need for compliance with stringent regulations. Organizations are increasingly recognizing that outsourcing can enhance efficiency and reduce the risk of non-compliance, thereby fostering a more robust healthcare regulatory-affairs-outsourcing market.
Increased Focus on Compliance and Risk Management
As regulatory scrutiny intensifies, organizations are placing a greater emphasis on compliance and risk management within the healthcare regulatory-affairs-outsourcing market. Companies are increasingly aware of the potential repercussions of non-compliance, which can include hefty fines and reputational damage. This heightened awareness is driving demand for outsourcing services that specialize in compliance management. The market is expected to expand as organizations seek to mitigate risks associated with regulatory failures. By outsourcing these functions, companies can benefit from the expertise of professionals who are well-versed in the latest regulations, thereby enhancing their compliance posture and contributing to the overall growth of the healthcare regulatory-affairs-outsourcing market.
Technological Advancements in Regulatory Processes
Technological innovations are transforming the landscape of the healthcare regulatory-affairs-outsourcing market. The integration of advanced technologies, such as artificial intelligence and data analytics, is streamlining regulatory processes and enhancing efficiency. These technologies enable organizations to analyze vast amounts of data quickly, improving decision-making and compliance tracking. As companies adopt these tools, the demand for outsourcing regulatory affairs is expected to rise, as specialized firms can provide the necessary technological expertise. This shift towards technology-driven solutions is likely to propel the growth of the healthcare regulatory-affairs-outsourcing market, as organizations seek to leverage these advancements to stay competitive and compliant.
Expansion of Biopharmaceutical and Medical Device Industries
The biopharmaceutical and medical device sectors are experiencing rapid growth, which is positively impacting the healthcare regulatory-affairs-outsourcing market. As these industries expand, the demand for regulatory expertise increases, particularly in navigating the complex approval processes for new products. The biopharmaceutical market alone is projected to reach $600 billion by 2025, necessitating robust regulatory support. Companies are increasingly turning to outsourcing to manage the regulatory demands associated with product development and market entry. This trend is likely to continue, as the healthcare regulatory-affairs-outsourcing market adapts to the needs of these burgeoning industries, ensuring compliance and facilitating timely product launches.






















Leave a Comment