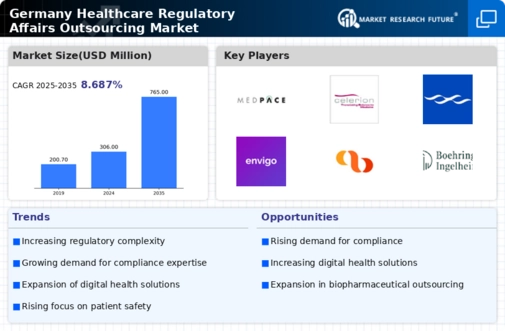
Increased Focus on Patient Safety
Patient safety remains a paramount concern within the healthcare sector, influencing the healthcare regulatory-affairs-outsourcing market. In Germany, regulatory bodies are intensifying their scrutiny of healthcare products and services to ensure they meet safety standards. This heightened focus is prompting companies to outsource their regulatory affairs to specialized firms that can provide the necessary expertise in compliance and safety assessments. The market is expected to witness a growth rate of around 12% in 2025, as organizations prioritize patient safety and seek to align with stringent regulatory requirements. By outsourcing, companies can leverage the knowledge of experts who are well-versed in the latest safety regulations, thereby enhancing their operational efficiency and compliance in the healthcare regulatory-affairs-outsourcing market.
Adoption of Digital Health Solutions
The integration of digital health solutions is transforming the healthcare landscape in Germany, thereby influencing the healthcare regulatory-affairs-outsourcing market. As telemedicine, mobile health applications, and electronic health records become more prevalent, regulatory requirements are evolving to address these innovations. Companies are increasingly outsourcing their regulatory affairs to adapt to these changes and ensure compliance with new digital health regulations. The market is projected to grow by approximately 10% in 2025, driven by the need for organizations to stay abreast of the rapidly changing regulatory environment. This trend indicates a growing reliance on specialized regulatory firms that can provide insights into the complexities of digital health compliance, thereby enhancing the overall effectiveness of the healthcare regulatory-affairs-outsourcing market.
Expansion of Biopharmaceutical Sector
The biopharmaceutical sector in Germany is undergoing rapid expansion, which is significantly impacting the healthcare regulatory-affairs-outsourcing market. As new biopharmaceutical products are developed, the need for comprehensive regulatory support becomes increasingly critical. In 2025, the biopharmaceutical market is anticipated to grow by 20%, leading to a corresponding rise in demand for regulatory affairs outsourcing. Companies are likely to seek external expertise to navigate the complex approval processes and ensure compliance with regulatory standards. This trend suggests that the healthcare regulatory-affairs-outsourcing market will play a crucial role in facilitating the successful launch of innovative biopharmaceutical products, thereby contributing to the overall growth of the healthcare industry.
Rising Complexity of Regulatory Frameworks
The healthcare regulatory-affairs-outsourcing market is experiencing a notable increase in the complexity of regulatory frameworks in Germany. This complexity arises from the evolving nature of healthcare laws, which necessitates specialized knowledge and expertise. As regulations become more intricate, companies are increasingly outsourcing their regulatory affairs to ensure compliance. In 2025, the market is projected to grow by approximately 15%, driven by the need for organizations to navigate these multifaceted regulations effectively. The demand for regulatory consultants and outsourcing services is likely to rise, as firms seek to mitigate risks associated with non-compliance. This trend indicates a shift towards a more collaborative approach in managing regulatory challenges, thereby enhancing the overall efficiency of the healthcare regulatory-affairs-outsourcing market.
Growing Demand for Market Access Strategies
In the competitive landscape of the healthcare sector, the demand for effective market access strategies is becoming increasingly pronounced, impacting the healthcare regulatory-affairs-outsourcing market. Companies are recognizing the importance of not only meeting regulatory requirements but also ensuring that their products gain timely access to the market. In 2025, the market is expected to grow by around 14%, as organizations seek to outsource their regulatory affairs to experts who can navigate the complexities of market access. This trend suggests that firms are prioritizing strategic partnerships with regulatory consultants to enhance their market positioning and compliance. By leveraging external expertise, companies can streamline their regulatory processes, thereby improving their chances of successful product launches in the healthcare regulatory-affairs-outsourcing market.

















Leave a Comment