The healthcare regulatory-affairs-outsourcing market in the UK is characterized by a competitive landscape that is increasingly shaped by innovation, strategic partnerships, and digital transformation. Key players such as IQVIA (US), Covance (US), and Parexel (US) are actively positioning themselves to leverage these dynamics. For instance, IQVIA (US) has focused on integrating advanced analytics and real-world evidence into its service offerings, thereby enhancing its value proposition to clients. Similarly, Covance (US) has emphasized its capabilities in clinical trial management and regulatory compliance, which are critical in navigating the complex healthcare landscape. These strategies collectively foster a competitive environment that prioritizes agility and responsiveness to regulatory changes.
In terms of business tactics, companies are increasingly localizing their operations to better serve regional markets, optimizing supply chains to enhance efficiency, and investing in technology to streamline processes. The market appears moderately fragmented, with a mix of large multinational corporations and smaller specialized firms. This structure allows for a diverse range of services and expertise, although the influence of major players remains substantial, often setting benchmarks for quality and innovation.
In October 2025, Parexel (US) announced a strategic partnership with a leading technology firm to develop AI-driven solutions for regulatory submissions. This move is significant as it positions Parexel (US) at the forefront of digital transformation within the regulatory space, potentially reducing submission times and improving accuracy. The integration of AI into regulatory processes could redefine operational efficiencies and client service delivery.
In September 2025, Covance (US) expanded its European operations by acquiring a local regulatory consultancy. This acquisition is indicative of Covance's (US) strategy to enhance its regional expertise and service offerings, allowing it to better navigate the intricacies of European regulations. Such expansions not only bolster Covance's (US) market presence but also enhance its ability to provide tailored solutions to clients facing diverse regulatory challenges.
In August 2025, IQVIA (US) launched a new platform aimed at streamlining the regulatory submission process for biopharmaceutical companies. This initiative reflects a broader trend towards digitalization in the industry, as companies seek to leverage technology to improve compliance and operational efficiency. The platform's introduction is likely to enhance IQVIA's (US) competitive edge by offering clients innovative tools that facilitate faster and more efficient regulatory processes.
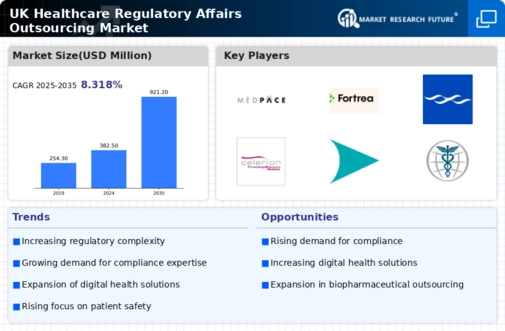
As of November 2025, the competitive trends in the healthcare regulatory-affairs-outsourcing market are increasingly defined by digitalization, sustainability, and the integration of AI technologies. Strategic alliances are becoming more prevalent, enabling companies to pool resources and expertise to address complex regulatory challenges. Looking ahead, it is anticipated that competitive differentiation will evolve, shifting from traditional price-based competition to a focus on innovation, technological advancement, and supply chain reliability. This transition underscores the importance of adaptability and forward-thinking strategies in maintaining a competitive advantage.






















Leave a Comment