Growing Awareness and Education
The growing awareness and education surrounding cardiovascular health in Italy are pivotal in shaping the structural heart-devices market. Public health campaigns and initiatives aimed at educating the population about heart disease risks and prevention strategies have gained momentum. As individuals become more informed about the importance of early detection and treatment, the demand for structural heart devices is expected to rise. Healthcare professionals are also increasingly emphasizing the role of these devices in managing heart conditions, leading to greater acceptance among patients. Additionally, collaborations between medical institutions and educational organizations are fostering a culture of knowledge sharing, which may further enhance the understanding of structural heart devices. This heightened awareness is likely to translate into increased patient inquiries and referrals for these innovative solutions, thereby positively impacting market growth.
Increased Healthcare Expenditure
The rising healthcare expenditure in Italy is a crucial factor propelling the structural heart-devices market. Recent reports indicate that healthcare spending in Italy has reached approximately €200 billion annually, with a significant portion allocated to cardiovascular care. This increase in funding allows for the procurement of advanced medical technologies, including structural heart devices, which are essential for treating complex heart conditions. Additionally, the Italian government has been investing in healthcare infrastructure, aiming to enhance the quality of care provided to patients. As hospitals and clinics upgrade their facilities and equipment, the demand for innovative structural heart devices is likely to grow. Furthermore, the emphasis on improving patient outcomes and reducing hospital readmission rates may lead to a greater focus on these devices, thereby driving market growth in the coming years.
Technological Innovations in Device Design
Technological advancements in the design and functionality of structural heart devices are significantly influencing the market in Italy. Innovations such as minimally invasive procedures and enhanced imaging techniques are transforming how these devices are developed and utilized. For instance, the introduction of transcatheter aortic valve replacement (TAVR) has revolutionized treatment options for patients with aortic stenosis, leading to a surge in demand for such devices. The structural heart-devices market is expected to benefit from ongoing research and development efforts aimed at improving device efficacy and safety. Moreover, the integration of digital health technologies, such as remote monitoring and telemedicine, is likely to enhance patient management and follow-up care. As these innovations continue to emerge, they may drive further adoption of structural heart devices, ultimately contributing to market expansion in Italy.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies in Italy is emerging as a significant driver for the structural heart-devices market. The Italian Medicines Agency (AIFA) has been actively promoting the approval and reimbursement of novel medical devices, facilitating quicker access to advanced treatments for patients. This supportive regulatory environment encourages manufacturers to invest in research and development, leading to the introduction of cutting-edge structural heart devices. Moreover, streamlined approval processes may result in a broader range of options available to healthcare providers, enhancing patient care. As regulatory bodies continue to prioritize innovation, the structural heart-devices market is likely to experience accelerated growth. The collaboration between regulatory agencies and industry stakeholders may also foster a more dynamic market landscape, ultimately benefiting patients in need of these life-saving interventions.
Rising Prevalence of Cardiovascular Diseases
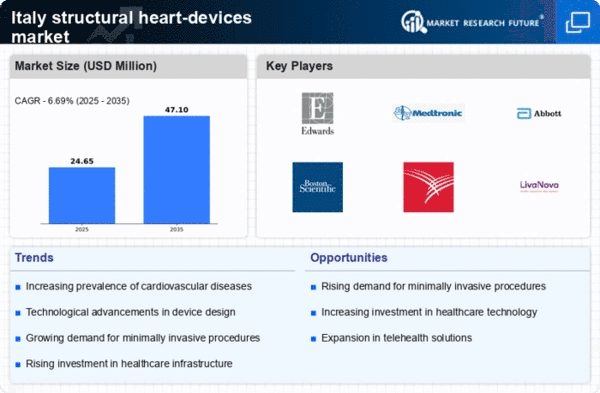
The increasing incidence of cardiovascular diseases in Italy is a primary driver for the structural heart-devices market. According to recent health statistics, cardiovascular diseases account for approximately 35% of all deaths in the country. This alarming trend necessitates advanced medical interventions, including structural heart devices, to manage and treat these conditions effectively. The demand for innovative solutions is further fueled by the aging population, which is more susceptible to heart-related ailments. As healthcare providers seek to improve patient outcomes, investments in structural heart devices are likely to rise, thereby expanding the market. Furthermore, the Italian healthcare system is increasingly prioritizing preventive care, which may lead to earlier interventions and a greater reliance on these devices. Consequently, the structural heart-devices market is poised for growth as healthcare professionals adapt to the evolving landscape of cardiovascular care.