Growing Awareness and Education
The rising awareness of heart health and the importance of early intervention is significantly impacting the structural heart-devices market in Germany. Public health campaigns and educational initiatives have been instrumental in informing both patients and healthcare professionals about the benefits of timely diagnosis and treatment of cardiovascular conditions. This heightened awareness is likely to lead to earlier detection of heart diseases, resulting in increased demand for structural heart devices. Additionally, healthcare providers are increasingly prioritizing patient education, which fosters a more proactive approach to heart health. As patients become more informed about their treatment options, the structural heart-devices market is expected to experience growth driven by informed decision-making and a greater willingness to pursue advanced medical interventions.
Increased Healthcare Expenditure
Germany's commitment to healthcare spending plays a crucial role in the growth of the structural heart-devices market. The country allocates a substantial portion of its GDP to healthcare, with expenditures reaching approximately 11.7% in recent years. This financial investment facilitates access to cutting-edge medical technologies, including structural heart devices. As hospitals and clinics receive funding for advanced equipment and training, the adoption of these devices is likely to increase. Furthermore, the German healthcare system emphasizes quality care, which aligns with the use of innovative devices that enhance patient outcomes. The combination of increased healthcare expenditure and a focus on advanced treatment options positions the structural heart-devices market for sustained growth in the foreseeable future.
Technological Innovations in Device Design
Innovations in the design and functionality of structural heart devices are transforming the landscape of cardiac care in Germany. The introduction of minimally invasive procedures and advanced materials has enhanced the efficacy and safety of these devices. For instance, the development of transcatheter aortic valve replacement (TAVR) systems has revolutionized treatment for aortic stenosis, leading to a surge in procedures performed. Market data indicates that the TAVR segment alone is projected to grow at a CAGR of over 15% in the coming years. These technological advancements not only improve patient outcomes but also reduce recovery times, making them more appealing to both patients and healthcare providers. As the structural heart-devices market continues to evolve, ongoing research and development efforts are expected to yield even more innovative solutions, further driving market growth.
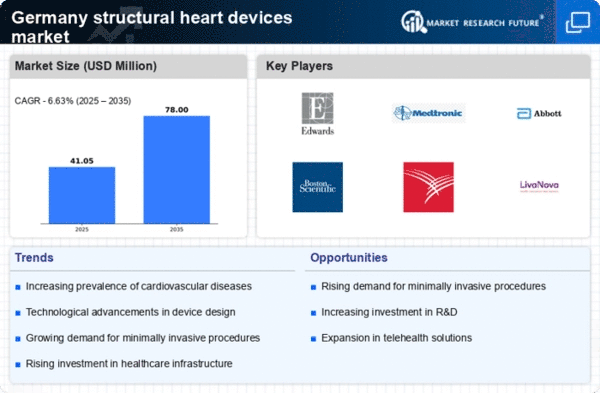
Rising Prevalence of Cardiovascular Diseases
The increasing incidence of cardiovascular diseases in Germany is a primary driver for the structural heart-devices market. According to recent health statistics, cardiovascular diseases account for approximately 40% of all deaths in the country. This alarming trend necessitates advanced medical interventions, including structural heart devices, to manage and treat various heart conditions effectively. The aging population, coupled with lifestyle factors such as obesity and diabetes, further exacerbates this issue. As healthcare providers seek innovative solutions to address these challenges, the demand for structural heart devices is expected to rise significantly. This growing need for effective treatment options is likely to propel the market forward, as patients and healthcare systems alike prioritize improved outcomes and quality of life through advanced medical technologies.
Regulatory Framework and Reimbursement Policies
The regulatory environment in Germany is conducive to the growth of the structural heart-devices market. The country has established a robust framework that supports the approval and commercialization of innovative medical devices. Regulatory bodies, such as the Federal Institute for Drugs and Medical Devices (BfArM), ensure that new products meet stringent safety and efficacy standards. Furthermore, favorable reimbursement policies for structural heart devices encourage healthcare providers to adopt these technologies. The German healthcare system's willingness to reimburse advanced treatments enhances accessibility for patients, thereby driving demand. As regulatory processes continue to evolve and adapt to technological advancements, the structural heart-devices market is likely to benefit from increased product availability and acceptance within the healthcare community.