Regulatory Support and Approval
Regulatory bodies are increasingly supportive of the development and approval of new therapies for hereditary angioedema, which serves as a significant driver for the Hereditary Angioedema Therapeutics Market. Streamlined approval processes and incentives for orphan drugs have encouraged pharmaceutical companies to invest in HAE therapeutics. The recent approvals of several new treatments have not only expanded the available options for patients but have also instilled confidence in the market. This regulatory environment is conducive to innovation, allowing for faster access to life-saving therapies. As a result, the Hereditary Angioedema Therapeutics Market is likely to experience accelerated growth, with more products entering the market in the coming years.
Advancements in Therapeutic Options
Technological advancements in the development of therapeutics are driving the Hereditary Angioedema Therapeutics Market. The introduction of novel therapies, including C1-inhibitor replacement therapies and bradykinin receptor antagonists, has transformed treatment paradigms. These innovations not only improve patient outcomes but also enhance the quality of life for individuals suffering from HAE. The market is witnessing a surge in research and development activities, with several new products entering the pipeline. For instance, recent data indicates that the market for HAE therapeutics is expected to reach USD 2 billion by 2026, underscoring the impact of these advancements on the industry. As more effective and targeted therapies become available, the landscape of the Hereditary Angioedema Therapeutics Market is likely to evolve rapidly.
Rising Incidence of Hereditary Angioedema
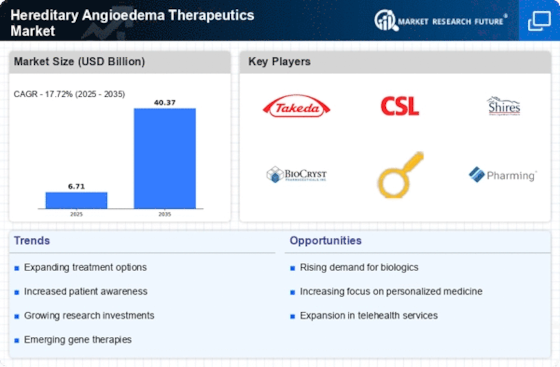
The increasing prevalence of hereditary angioedema (HAE) is a notable driver for the Hereditary Angioedema Therapeutics Market. Recent estimates suggest that HAE affects approximately 1 in 10,000 to 1 in 50,000 individuals, leading to a growing patient population in need of effective treatments. This rise in incidence is prompting healthcare providers and pharmaceutical companies to focus on developing innovative therapies. As awareness of HAE expands, more patients are being diagnosed, which in turn fuels demand for specialized therapeutics. The market is projected to grow significantly, with a compound annual growth rate (CAGR) of around 8% over the next several years, reflecting the urgent need for effective management options in the Hereditary Angioedema Therapeutics Market.
Growing Patient Advocacy and Support Groups
The rise of patient advocacy and support groups is significantly influencing the Hereditary Angioedema Therapeutics Market. These organizations play a vital role in raising awareness about HAE, educating patients, and advocating for better treatment options. Their efforts have led to increased visibility of the condition, prompting healthcare providers to prioritize HAE management. Furthermore, these groups often collaborate with pharmaceutical companies to facilitate clinical trials and gather patient feedback, which can enhance therapeutic development. As patient engagement continues to grow, the demand for effective treatments is likely to increase, thereby driving the Hereditary Angioedema Therapeutics Market forward.
Increased Investment in Research and Development
Investment in research and development (R&D) for hereditary angioedema therapies is a crucial driver for the Hereditary Angioedema Therapeutics Market. Pharmaceutical companies are allocating substantial resources to explore new treatment modalities and improve existing therapies. This focus on R&D is driven by the unmet medical needs of HAE patients and the potential for lucrative returns on investment. Recent reports indicate that R&D spending in the HAE sector is expected to increase by over 15% annually, reflecting the industry's commitment to innovation. As more companies enter the market and competition intensifies, the landscape of the Hereditary Angioedema Therapeutics Market is poised for transformation, with a plethora of new options for patients.