Market Analysis
In-depth Analysis of Hereditary Angioedema Therapeutics Market Industry Landscape
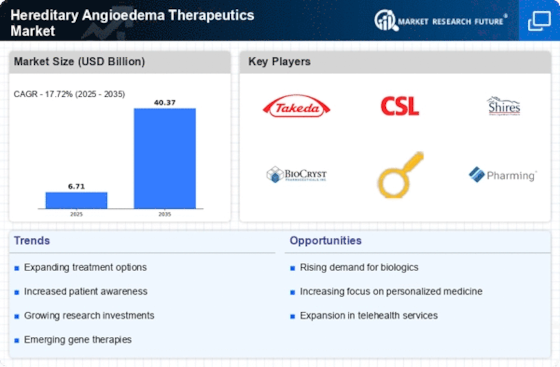
The HAE Therapeutics Market shows a distinctive dynamics that are generated from a combination of multiple factors existing within these industries. The primary significance of this market is the overwhelming existence and awareness of hereditary angioedema, an exceedingly rare genetic disorder that is manifested by frequent and unpredictable swelling episodes. The HAE awareness and diagnosis improvement progresses the demand for practical therapeutic options. Highly variable incidences of HAE by region will determine the global distribution of the disease.
Medical research and technology developments in this direction influence the HAE therapeutics markets dynamics. Frequent investigations of the genetic and biochemical causal factors allow to develop specific therapy directed to the cause of hereditary angioedema. Biotechnological innovations, notably the development of recombinant proteins and monoclonal antibodies, increase the treatment options for patients with hereditary angioedema (HAE), consequently leading to more market dynamics.
Regulatory considerations comprise essential factors when considering therapeutic development and commercialization for hereditary angioedema. Under strict regulatory standards, people in the healthcare profession and patients have total faith in the efficacy, safety and quality of the HAE medicine. Regulatory requirement commitment creates market competition whereby pharmaceutical companies navigate the regulatory environment to bring their specific HAE drug to market.
Economic variable are pivotal in the aspect of HAE therapeutics market. Economic development and stability in turn lead to more spending on healthcare, and as a result, HAE patients find it easier to access their treatments. On the other hand, the economic problems may limit health budget, directing the accessibility and affordability of the inherited angioedema treatment. Of course, the economic aspect is also formed by the development of more and more cost-effective ways of treatment and expansion of high-tech medicine.
The pharmaceutical companies, biotech firms, and research institutions are in competition in the market for another significant market factor. Intense rivalry is in the forefront and companies are always ready to come up with advanced therapeutic entities and treatment options that are able to deal with hereditary angioedema. Collaborations, partnerships, and clinical trials are the three major elements that influence the competitive landscape as companies work to improve their R&D activities, product portfolios, and gain an advantage within the HAE therapeutics market.
The demands of healthcare professionals, patients and researchers form the basis upon which HAE therapeutics market is determined. In choosing a specific treatment modality, physicians and pharmacists may look for evidence of effectiveness, safety and practicality. The patients may prefer the therapies that offer better symptom control, less treatedysed burden as well as higher quality of life.

















Leave a Comment