Growing Awareness and Education
There is a notable increase in awareness and education regarding cardiovascular health in France, which is positively impacting the structural heart-devices market. Public health campaigns and initiatives aimed at educating the population about heart disease risks and prevention strategies are becoming more prevalent. This heightened awareness is leading to earlier diagnosis and treatment of cardiovascular conditions, thereby increasing the demand for structural heart devices. Healthcare professionals are also receiving enhanced training on the latest technologies and treatment options, further driving the market. As patients become more informed about their treatment choices, the structural heart-devices market is likely to experience growth, reflecting a shift towards proactive healthcare management.
Increased Healthcare Expenditure
The rise in healthcare expenditure in France is a significant driver for the structural heart-devices market. The French government has been increasing its healthcare budget, with spending projected to reach approximately €200 billion by 2025. This financial commitment reflects a prioritization of advanced medical technologies and treatments, particularly in the field of cardiology. As hospitals and healthcare facilities receive more funding, they are likely to invest in state-of-the-art structural heart devices to enhance patient care. This trend not only supports the growth of the market but also encourages competition among manufacturers to provide innovative solutions. Consequently, the structural heart-devices market is poised for expansion, driven by the increasing availability of resources and a focus on improving healthcare outcomes.
Supportive Regulatory Environment
The regulatory environment in France is becoming increasingly supportive of innovations in the structural heart-devices market. Regulatory bodies are streamlining approval processes for new medical devices, which facilitates quicker access to advanced treatments for patients. This supportive framework encourages manufacturers to invest in research and development, knowing that their products can reach the market more efficiently. Additionally, the French government is actively promoting initiatives that foster collaboration between industry stakeholders and regulatory agencies, enhancing the overall ecosystem for medical device innovation. As a result, the structural heart-devices market is expected to benefit from a more favorable regulatory landscape, which could lead to an influx of new products and technologies in the coming years.
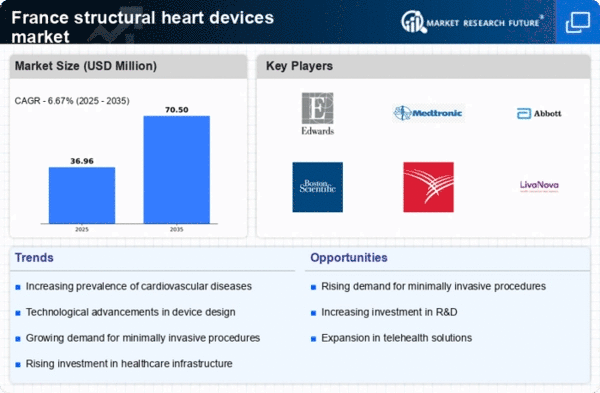
Rising Prevalence of Cardiovascular Diseases
The increasing incidence of cardiovascular diseases in France is a primary driver for the structural heart-devices market. According to recent health statistics, cardiovascular diseases account for approximately 30% of all deaths in the country. This alarming trend necessitates advanced medical interventions, including structural heart devices, to manage and treat these conditions effectively. The demand for innovative solutions is further fueled by the aging population, which is more susceptible to heart-related ailments. As healthcare providers seek to improve patient outcomes, investments in the structural heart-devices market are likely to rise, reflecting a growing recognition of the need for effective treatment options. This trend indicates a robust market potential, as healthcare systems adapt to the increasing burden of cardiovascular diseases.
Technological Innovations in Medical Devices
Technological advancements in medical devices are significantly influencing the structural heart-devices market. Innovations such as minimally invasive procedures and advanced imaging techniques are enhancing the efficacy and safety of heart surgeries. For instance, the development of transcatheter aortic valve replacement (TAVR) has revolutionized treatment for aortic stenosis, allowing for reduced recovery times and improved patient outcomes. The French market is witnessing a surge in the adoption of these technologies, with a projected growth rate of around 8% annually. This growth is indicative of the healthcare sector's commitment to integrating cutting-edge technologies into patient care, thereby driving the demand for structural heart devices. As manufacturers continue to invest in research and development, the market is expected to expand further, offering a range of innovative solutions for cardiovascular treatment.