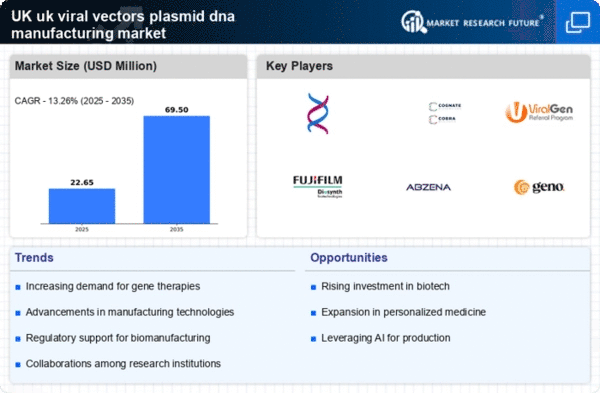
Market Growth Projections
The Global UK Viral Vectors and Plasmid DNA Manufacturing Market Industry is poised for substantial growth, with projections indicating a rise from 3.5 USD Billion in 2024 to 9.2 USD Billion by 2035. This growth trajectory suggests a compound annual growth rate of 9.18% from 2025 to 2035, reflecting increasing investments in research and development, technological advancements, and a growing demand for gene therapies. The market's expansion is likely to be driven by the convergence of various factors, including regulatory support, rising chronic disease prevalence, and advancements in manufacturing technologies. These elements collectively contribute to a robust outlook for the industry.
Growing Demand for Gene Therapies
The Global UK Viral Vectors and Plasmid DNA Manufacturing Market Industry is experiencing a notable surge in demand for gene therapies. This increase is largely driven by advancements in genetic engineering and the rising prevalence of genetic disorders. As of 2024, the market is valued at approximately 3.5 USD Billion, reflecting a robust interest in innovative treatment options. The potential for viral vectors to deliver therapeutic genes effectively positions them as a cornerstone in the development of next-generation therapies. This trend is expected to continue, with the market projected to reach 9.2 USD Billion by 2035, indicating a strong growth trajectory.
Rising Prevalence of Chronic Diseases
The Global UK Viral Vectors and Plasmid DNA Manufacturing Market Industry is significantly influenced by the rising prevalence of chronic diseases, which necessitates innovative treatment solutions. Conditions such as cancer, diabetes, and genetic disorders are becoming increasingly common, driving the demand for effective therapies. Viral vectors and plasmid DNA are pivotal in developing targeted treatments that address these health challenges. As the market evolves, the need for advanced therapeutic options is expected to grow, contributing to the projected compound annual growth rate of 9.18% from 2025 to 2035. This trend underscores the critical role of viral vectors and plasmid DNA in addressing global health concerns.
Increasing Investment in Biotechnology
Investment in biotechnology is a key driver for the Global UK Viral Vectors and Plasmid DNA Manufacturing Market Industry. Venture capital and public funding are increasingly directed towards companies specializing in gene therapies and related technologies. This influx of capital enables firms to enhance their research capabilities, expand production facilities, and accelerate the development of novel therapies. As the market is projected to grow from 3.5 USD Billion in 2024 to 9.2 USD Billion by 2035, the role of investment in fostering innovation and scaling operations cannot be overstated. The growing interest from investors reflects a broader recognition of the potential returns associated with advancements in this field.
Regulatory Support for Biopharmaceuticals
The Global UK Viral Vectors and Plasmid DNA Manufacturing Market Industry benefits from a favorable regulatory environment that supports the development and commercialization of biopharmaceuticals. Regulatory agencies are increasingly recognizing the potential of gene therapies and are streamlining approval processes to facilitate quicker market entry. This supportive framework encourages investment in research and development, fostering innovation within the sector. As the market evolves, regulatory clarity is likely to enhance confidence among stakeholders, thereby driving growth. The anticipated expansion of the market to 9.2 USD Billion by 2035 suggests that regulatory support will play a crucial role in its trajectory.
Advancements in Manufacturing Technologies
Technological advancements in the manufacturing processes of viral vectors and plasmid DNA are significantly influencing the Global UK Viral Vectors and Plasmid DNA Manufacturing Market Industry. Innovations such as continuous manufacturing and improved purification techniques enhance yield and reduce production costs. These advancements not only streamline the manufacturing process but also ensure higher quality and consistency of the final products. As a result, companies are better positioned to meet the increasing demand for high-quality viral vectors and plasmid DNA. The anticipated compound annual growth rate of 9.18% from 2025 to 2035 further underscores the importance of these technological developments in shaping the market.






















Leave a Comment