Market Growth Projections
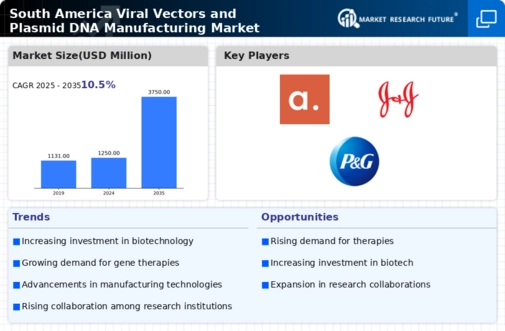
The Global South America Viral Vectors and Plasmid DNA Manufacturing Market Industry is on a growth trajectory, with projections indicating a substantial increase in market size. The market is expected to reach 1250 USD Million in 2024 and is forecasted to expand to 3750 USD Million by 2035. This growth reflects a compound annual growth rate of 10.5% from 2025 to 2035, driven by factors such as technological advancements, increasing demand for gene therapies, and supportive regulatory frameworks. The market's expansion is indicative of the growing importance of viral vectors and plasmid DNA in the biopharmaceutical landscape.
Rising Demand for Gene Therapy
The Global South America Viral Vectors and Plasmid DNA Manufacturing Market Industry experiences a surge in demand for gene therapy solutions. As advancements in genetic engineering continue to evolve, the need for efficient viral vectors and plasmid DNA is paramount. In 2024, the market is projected to reach 1250 USD Million, driven by increasing investments in research and development. This trend indicates a growing recognition of gene therapy's potential to treat genetic disorders and chronic diseases, thereby expanding the market's scope. By 2035, the market is expected to grow to 3750 USD Million, reflecting a compound annual growth rate of 10.5% from 2025 to 2035.
Supportive Regulatory Framework
A supportive regulatory environment is vital for the Global South America Viral Vectors and Plasmid DNA Manufacturing Market Industry. Governments in South America are increasingly recognizing the importance of biopharmaceuticals and are implementing policies to facilitate research and development. This includes streamlined approval processes for gene therapies and enhanced funding for biotechnology initiatives. Such measures not only encourage innovation but also attract investments into the sector. As a result, the market is likely to experience robust growth, with expectations of reaching 3750 USD Million by 2035, fueled by a favorable regulatory landscape.
Growing Investment in Biotechnology
The Global South America Viral Vectors and Plasmid DNA Manufacturing Market Industry benefits from a surge in investment in biotechnology. Venture capital and public funding are increasingly directed towards biopharmaceutical research, particularly in gene therapy and personalized medicine. This influx of capital enables companies to enhance their manufacturing capabilities and expand their product offerings. As the market evolves, it is anticipated that the financial backing will support the growth trajectory, with projections indicating a rise from 1250 USD Million in 2024 to 3750 USD Million by 2035, reflecting a compound annual growth rate of 10.5% from 2025 to 2035.
Increasing Prevalence of Genetic Disorders
The rising prevalence of genetic disorders significantly impacts the Global South America Viral Vectors and Plasmid DNA Manufacturing Market Industry. As awareness of genetic conditions grows, there is an increasing demand for effective treatment options, particularly gene therapies. This trend is prompting pharmaceutical companies to invest in the development of viral vectors and plasmid DNA, which are essential for delivering therapeutic genes. The market is projected to grow from 1250 USD Million in 2024 to 3750 USD Million by 2035, driven by the urgent need for innovative solutions to address genetic disorders.
Technological Advancements in Manufacturing
Technological innovations play a crucial role in the Global South America Viral Vectors and Plasmid DNA Manufacturing Market Industry. The introduction of advanced bioprocessing techniques and automation enhances production efficiency and scalability. These improvements not only reduce costs but also ensure higher quality and consistency in the final products. As companies adopt these technologies, they can meet the increasing demand for viral vectors and plasmid DNA, which are essential for various therapeutic applications. Consequently, the market is poised for significant growth, with projections indicating a rise from 1250 USD Million in 2024 to 3750 USD Million by 2035.