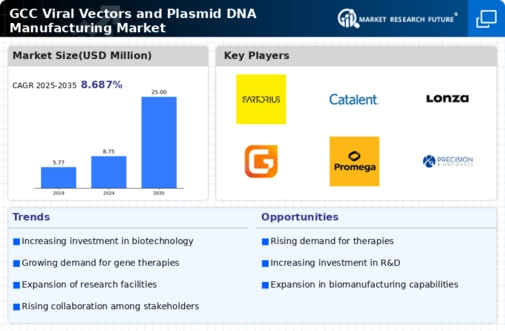
The GCC Viral Vectors and Plasmid DNA Manufacturing Market is experiencing significant growth due to increasing investments in biotechnology and genetic research. This distinctive market is characterized by a competitive landscape featuring numerous companies capitalizing on the growing demand for viral vectors and plasmid DNA in therapeutic applications, particularly in gene therapies and vaccine development. The market dynamics are shaped by advancements in manufacturing technologies, regulatory frameworks, and the continual rise in healthcare needs driven by public health challenges.
The ability to provide high-quality, scalable solutions in vector and plasmid production offers companies a competitive edge critical for establishing market dominance in this niche field.Sartorius has established a notable presence in the GCC Viral Vectors and Plasmid DNA Manufacturing Market, leveraging its robust capabilities in bioprocessing solutions. Their strengths lie in their innovative technologies and comprehensive service offerings that facilitate efficient manufacturing processes and ensure compliance with stringent regulatory standards. Sartorius is known for integrating well-designed modular equipment and advanced analytics tools that optimize yield and quality throughout the production lifecycle.
Their commitment to research and development fuels their growth trajectory in the region, and they strategically target collaborations with local biotech firms to strengthen their market foothold, thus enhancing their competitive advantage in the ever-evolving landscape of viral vector and plasmid manufacturing.Catalent is another major player in the GCC Viral Vectors and Plasmid DNA Manufacturing Market, recognized for its comprehensive portfolio that includes pivotal manufacturing services essential for gene therapies and vaccines. The company excels in providing tailored solutions, including development and commercialization support, which positions it favorably within the regional market.
Catalent’s strengths are amplified by their extensive manufacturing capabilities and a network of facilities that adhere to global quality standards. The company has also been active in pursuing strategic partnerships and acquisitions that bolster its position in the GCC, increasing its ability to meet local demand effectively. By integrating advanced technologies such as their proprietary gene delivery platform, Catalent not only enhances its service offerings but also demonstrates a commitment to innovation, ensuring it remains a strong contender in the competitive landscape of viral vectors and plasmid DNA manufacturing in the GCC region.
























Leave a Comment