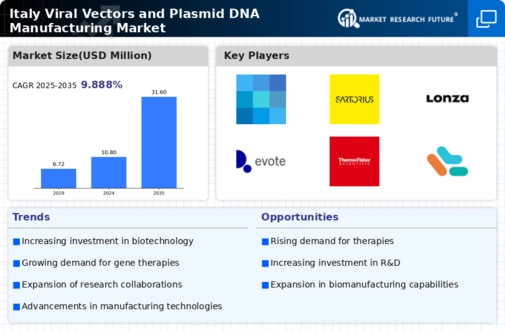
Growing Demand for Gene Therapies
The increasing prevalence of genetic disorders and chronic diseases in Italy is driving the demand for innovative treatment solutions, particularly gene therapies. The Italy viral vectors plasmid DNA manufacturing market is witnessing a surge in interest as healthcare providers and patients seek effective therapies. According to recent data, the Italian biotechnology sector has seen a growth rate of approximately 8% annually, indicating a robust market for gene therapies. This trend is likely to continue as advancements in genetic research and technology pave the way for new treatment modalities, further propelling the demand for viral vectors and plasmid DNA manufacturing.
Supportive Regulatory Environment
Italy's regulatory framework is becoming increasingly supportive of gene therapy development, which is a crucial driver for the Italy viral vectors plasmid DNA manufacturing market. The Italian Medicines Agency (AIFA) has streamlined approval processes for gene therapies, facilitating faster access to innovative treatments. This regulatory support not only encourages investment in research and development but also enhances collaboration between public and private sectors. As a result, the market is expected to expand, with more companies entering the field to capitalize on the favorable regulatory landscape, thereby boosting the production of viral vectors and plasmid DNA.
Increased Public and Private Investment
The influx of public and private investment in the biotechnology sector is a key driver for the Italy viral vectors plasmid DNA manufacturing market. Government initiatives aimed at fostering innovation and supporting research in gene therapies have led to substantial funding opportunities for biotech firms. For instance, the Italian government has allocated significant resources to support research projects focused on gene therapy development. This financial backing not only stimulates growth within the industry but also encourages collaboration between academic institutions and biotech companies, further enhancing the capabilities of the viral vectors and plasmid DNA manufacturing sector.
Technological Advancements in Manufacturing
Recent technological advancements in the manufacturing processes of viral vectors and plasmid DNA are significantly impacting the Italy viral vectors plasmid DNA manufacturing market. Innovations such as improved transfection techniques and enhanced purification methods are leading to higher yields and better quality products. These advancements are crucial for meeting the growing demand for gene therapies and ensuring compliance with stringent regulatory standards. As Italian companies adopt these cutting-edge technologies, they are likely to enhance their competitive edge in the global market, thereby contributing to the overall growth of the industry.
Rising Awareness and Acceptance of Gene Therapies
There is a notable increase in awareness and acceptance of gene therapies among healthcare professionals and patients in Italy, which is positively influencing the Italy viral vectors plasmid DNA manufacturing market. Educational campaigns and successful case studies are helping to demystify gene therapies, leading to greater trust and willingness to adopt these innovative treatments. As more patients and healthcare providers recognize the potential benefits of gene therapies, the demand for viral vectors and plasmid DNA is expected to rise. This growing acceptance is likely to drive further investment and innovation within the industry, fostering a more robust market environment.