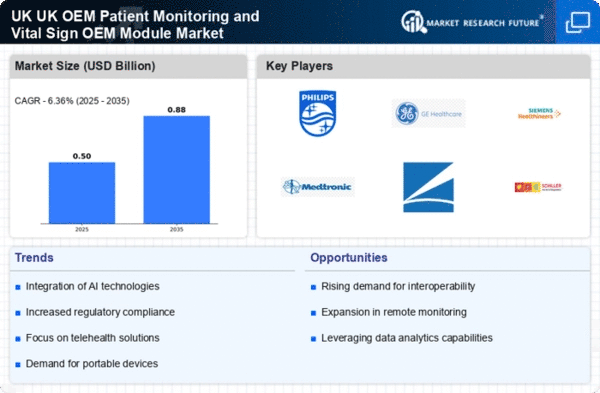
The UK OEM Patient Monitoring Vital Sign OEM Module Market has been evolving rapidly, driven by advancements in technology, increasing healthcare demands, and the push for better patient outcomes. This market includes a range of solutions designed for continuous monitoring of vital signs, such as heart rate, blood pressure, and oxygen levels, which are critical for patient care in various settings.
Competition in this sector is intense, with numerous players striving to innovate and deliver reliable products. The landscape is shaped by key factors, including regulatory compliance, technological advancements, and the ability to forge robust partnerships within the healthcare ecosystem.
Companies within this market leverage their strengths in research and development, distribution networks, and customer service to gain a competitive edge, all while navigating the complexities of healthcare regulations in the UK.
Siemens Healthineers
Siemens Healthineers holds a notable position in the UK OEM Patient Monitoring Vital Sign OEM Module Market. Renowned for its strong reputation in medical technology, the company has established a robust presence through its commitment to innovation and quality.
Siemens Healthineers offers a comprehensive range of vital sign monitoring solutions designed to enhance clinical workflows and improve patient monitoring accuracy. The company's strengths lie in its advanced technological capabilities, collaboration with healthcare providers, and a deep understanding of market needs.
This enables Siemens Healthineers to maintain a competitive advantage while fostering strong relationships with customers in the UK, ensuring that hospitals and clinics have access to the latest advancements in patient monitoring technology.
Osaka Pharmaceutical
Osaka Pharmaceutical, operating within the UK OEM Patient Monitoring Vital Sign OEM Module Market, focuses on delivering high-quality healthcare solutions tailored to the specific needs of healthcare providers. The company is known for its innovative products, including vital sign monitoring devices that integrate seamlessly into existing healthcare infrastructures.
Osaka Pharmaceutical's market presence is marked by a strong commitment to research and development, resulting in continuous improvements and updates to its offerings. The company has also engaged in strategic partnerships and possible mergers and acquisitions, which bolster its market position and expand its product portfolio.
In the UK, Osaka Pharmaceutical is recognized for its strengths in understanding local healthcare requirements, allowing it to provide customized solutions that address the unique challenges faced by healthcare facilities in monitoring patient vital signs effectively.




















Leave a Comment