Growing Aging Population
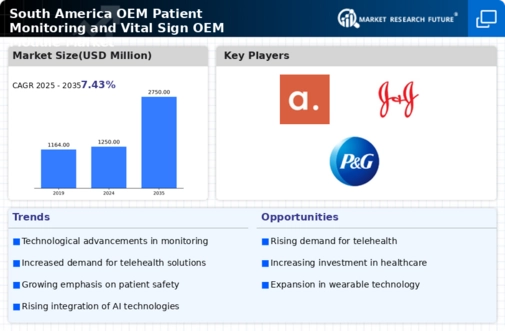
The demographic shift towards an aging population in South America is a crucial driver for the Global South America OEM Patient Monitoring and Vital Sign OEM Module Market Industry. As the elderly population increases, the demand for effective monitoring solutions to manage age-related health issues rises correspondingly. This demographic trend necessitates the implementation of advanced patient monitoring systems to ensure timely interventions and improve quality of life. The market's growth trajectory, projected to reach 2750 USD Million by 2035, underscores the importance of addressing the healthcare needs of this demographic. Consequently, the industry is likely to adapt to these changing needs by offering tailored monitoring solutions.
Market Growth Projections
The Global South America OEM Patient Monitoring and Vital Sign OEM Module Market Industry is poised for substantial growth, with projections indicating a market value of 1250 USD Million in 2024 and an anticipated increase to 2750 USD Million by 2035. This growth trajectory suggests a compound annual growth rate of 7.43% from 2025 to 2035, reflecting the increasing adoption of advanced monitoring technologies across healthcare settings. The market's expansion is likely to be driven by various factors, including technological advancements, demographic shifts, and a growing emphasis on preventive healthcare. These dynamics collectively indicate a promising outlook for the industry.
Government Initiatives and Funding
Government initiatives aimed at improving healthcare infrastructure significantly influence the Global South America OEM Patient Monitoring and Vital Sign OEM Module Market Industry. Various South American countries are investing in healthcare modernization, which includes the procurement of advanced patient monitoring systems. For example, public health programs are increasingly allocating funds for the acquisition of OEM modules that enhance patient care capabilities. This financial support is likely to stimulate market growth, as healthcare facilities upgrade their monitoring systems. The anticipated compound annual growth rate of 7.43% from 2025 to 2035 indicates a robust expansion driven by these government initiatives.
Increased Focus on Preventive Healthcare
The Global South America OEM Patient Monitoring and Vital Sign OEM Module Market Industry is witnessing a paradigm shift towards preventive healthcare. Healthcare providers are increasingly prioritizing early detection and management of health conditions, which drives the demand for effective monitoring solutions. This focus on prevention is reflected in the growing adoption of OEM modules that facilitate continuous monitoring of vital signs. As a result, the market is projected to grow at a compound annual growth rate of 7.43% from 2025 to 2035. This trend indicates a broader recognition of the value of proactive healthcare measures in improving patient outcomes and reducing healthcare costs.
Rising Demand for Remote Patient Monitoring
The Global South America OEM Patient Monitoring and Vital Sign OEM Module Market Industry experiences a notable surge in demand for remote patient monitoring solutions. This trend is driven by the increasing prevalence of chronic diseases, which necessitate continuous monitoring of patients' vital signs. For instance, the market is projected to reach 1250 USD Million in 2024, reflecting a growing recognition of the importance of remote monitoring in enhancing patient outcomes. The convenience and efficiency of these solutions are likely to attract healthcare providers, thereby fostering market growth. As healthcare systems evolve, the integration of advanced monitoring technologies appears essential for improving patient care.
Technological Advancements in Monitoring Devices
Technological innovations play a pivotal role in shaping the Global South America OEM Patient Monitoring and Vital Sign OEM Module Market Industry. The introduction of sophisticated monitoring devices equipped with advanced features, such as wireless connectivity and real-time data analytics, enhances the accuracy and reliability of patient monitoring. These advancements not only improve clinical decision-making but also facilitate timely interventions. As healthcare providers increasingly adopt these technologies, the market is expected to witness substantial growth, with projections indicating a rise to 2750 USD Million by 2035. The continuous evolution of monitoring technologies suggests a promising future for the industry.













Leave a Comment