OEM Patient Monitoring Vital Sign OEM Module Market Summary
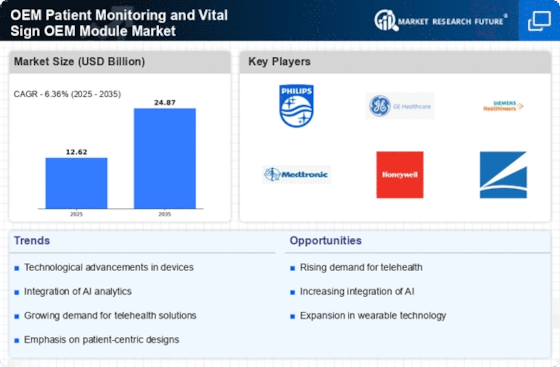
The Global OEM Patient Monitoring and Vital Sign OEM Module Market is projected to grow from 12.62 USD Billion in 2024 to 24.87 USD Billion by 2035.
Key Market Trends & Highlights
OEM Patient Monitoring and Vital Sign OEM Module Key Trends and Highlights
- The market is expected to experience a compound annual growth rate (CAGR) of 7.47 percent from 2025 to 2035.
- By 2035, the market valuation is anticipated to reach 27.9 USD Billion, indicating robust growth potential.
- in 2024, the market is valued at 12.62 USD Billion, reflecting a strong foundation for future expansion.
- Growing adoption of advanced monitoring technologies due to increasing demand for patient safety is a major market driver.
Market Size & Forecast
| 2024 Market Size | 12.62 (USD Billion) |
| 2035 Market Size | 24.87 (USD Billion) |
| CAGR (2025-2035) | 6.36% |
Major Players
Sentec AG (Switzerland), Medtronic Plc (Ireland), Nonin Medical Inc. (US), Masimo Corporation (US), Ronseda Electronics Co., Ltd (China), Triton Electronic Systems Ltd (Russia), Halma Plc (UK), Zug Medical System SAS (France), Fresenius Kabi AG (Germany), Smiths Group plc (UK)













Leave a Comment