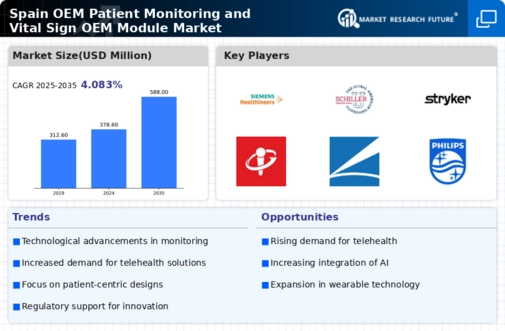
A number of significant trends are currently being observed in the Spain OEM Patient Monitoring and Vital Sign OEM Module Market. Advanced patient monitoring systems are becoming increasingly popular in hospitals and clinics throughout Spain, which is one of the primary market drivers. In an effort to improve the quality of patient care, the Spanish healthcare system is consistently seeking out innovations, particularly in critical care environments. As the population ages and the prevalence of chronic diseases increases, there is an increasing demand for monitoring solutions that are effective in increasing patient outcomes.
Spain's healthcare sector is prioritizing the seamless exchange of patient data across platforms and interoperability, in accordance with the European Union's initiative to advance digital health. In addition, local manufacturers are creating tailored solutions to satisfy the unique requirements of Spanish healthcare providers, ensuring that their products are in accordance with local market demands and regulatory standards.
Given the increasing emphasis on patient-centered care, regulatory support, and innovation, the OEM Patient Monitoring and Vital Sign OEM Module Market is well-positioned for future growth in Spain.
Spain OEM Patient Monitoring and Vital Sign OEM Module Market Driver
Increasing Aging Population in Spain
Spain has one of the highest proportions of elderly individuals in Europe, with over 19% of its population aged 65 and older, according to the National Institute of Statistics of Spain. This demographic shift significantly impacts the Spain OEM Patient Monitoring and Vital Sign OEM Module Market Industry, as older adults are more susceptible to chronic diseases that require continuous monitoring.
The Spanish government has been enhancing its healthcare infrastructure to cater to this aging population, which further drives the demand for advanced patient monitoring solutions. Established companies such as Philips and Siemens Healthineers are also investing in the development of innovative monitoring technologies tailored to elderly care, reinforcing the growth potential in this sector.
The result is a projected increase in demand for patient monitoring modules that can provide timely and accurate readings of vital signs, essential for managing the health of the elderly.
Rising Chronic Disease Incidence
The increasing prevalence of chronic diseases in Spain, such as diabetes and cardiovascular diseases, is a significant driver for the Spain OEM Patient Monitoring and Vital Sign OEM Module Market Industry. According to the Spanish Society of Cardiology, cardiovascular diseases affect approximately 27% of the population.
Organizations like the World Health Organization have reported a growing trend in lifestyle-related ailments, emphasizing the need for robust monitoring solutions. These chronic conditions often necessitate continuous monitoring, leading healthcare providers to invest in quality patient monitoring modules to enhance patient outcomes. Major players like GE Healthcare are tailoring their solutions to meet this emerging need, making them pivotal in expanding the market.
Technological Advancements in Healthcare
Rapid advancements in technology, particularly in remote monitoring and telehealth, are propelling the Spain OEM Patient Monitoring and Vital Sign OEM Module Market Industry forward. The Spanish government has been promoting digital health initiatives, which include the integration of remote patient monitoring to improve healthcare delivery, as highlighted in their national health strategy.
The COVID-19 pandemic accelerated the adoption of telehealth solutions, encouraging firms like Medtronic and IBM Watson Health to innovate and develop advanced monitoring modules that facilitate real-time health assessments. This trend not only enhances patient management but also offers healthcare providers the tools needed to streamline processes, thus contributing to an expanded market for OEM modules.
The evolution of technology in patient monitoring systems appears to enhance the accuracy and efficiency of vital sign measurements, thereby potentially improving patient outcomes in clinical settings.
Spanish Ministry of Health






















Leave a Comment