Globalization of Clinical Trials
The globalization of clinical trials is a significant driver for the healthcare regulatory-affairs-outsourcing market. As pharmaceutical companies expand their research efforts beyond national borders, they encounter diverse regulatory environments. In France, this trend necessitates a nuanced understanding of both local and international regulations, prompting firms to outsource regulatory affairs to experts familiar with these complexities. The demand for regulatory support in managing multi-regional trials is expected to increase, as companies aim to ensure compliance across various jurisdictions. By 2025, the healthcare regulatory-affairs-outsourcing market is likely to experience a growth rate of 11%, driven by the need for specialized knowledge in navigating the regulatory landscapes of multiple countries.
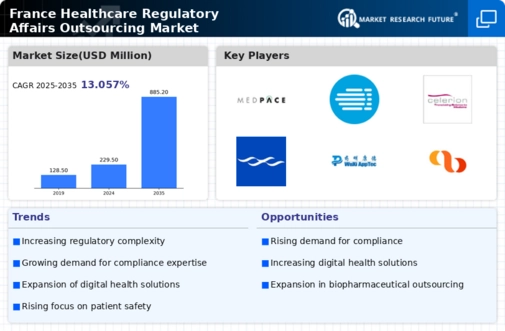
Rising Complexity of Regulations
The increasing complexity of healthcare regulations in France is a primary driver for the healthcare regulatory-affairs-outsourcing market. As regulatory frameworks evolve, companies face challenges in compliance, necessitating specialized expertise. This complexity is underscored by the European Medicines Agency's (EMA) ongoing updates to guidelines, which can lead to significant delays in product approvals if not navigated correctly. Consequently, organizations are increasingly outsourcing regulatory affairs to ensure adherence to these intricate regulations. The market is projected to grow as firms seek to mitigate risks associated with non-compliance, which can result in penalties and reputational damage. In 2025, the healthcare regulatory-affairs-outsourcing market is expected to witness a growth rate of approximately 12% in France, driven by the need for expert guidance in navigating these regulatory landscapes.
Increased Focus on Patient Safety
Patient safety remains a paramount concern within the healthcare sector, influencing the healthcare regulatory-affairs-outsourcing market. Regulatory bodies in France are intensifying their scrutiny of clinical trials and post-market surveillance to ensure that products meet safety standards. This heightened focus necessitates comprehensive regulatory strategies, prompting companies to outsource these functions to specialized firms. The demand for regulatory expertise in safety assessments is likely to increase, as organizations aim to enhance their compliance with evolving safety regulations. In 2025, the market is anticipated to expand as companies prioritize patient safety, with an estimated growth of 10% attributed to the outsourcing of regulatory affairs to ensure adherence to stringent safety protocols.
Cost Efficiency and Resource Optimization
Cost efficiency is a critical consideration for organizations operating in the healthcare sector, influencing the healthcare regulatory-affairs-outsourcing market. By outsourcing regulatory affairs, companies can reduce operational costs associated with maintaining in-house teams and infrastructure. This approach allows firms to allocate resources more effectively, focusing on core competencies while ensuring compliance with regulatory requirements. In France, the trend towards outsourcing is expected to accelerate as organizations seek to optimize their operational expenditures. The healthcare regulatory-affairs-outsourcing market is projected to grow by 9% in 2025, as companies recognize the financial benefits of leveraging external expertise to navigate the complexities of regulatory compliance.
Technological Advancements in Regulatory Processes
Technological advancements are reshaping the landscape of the healthcare regulatory-affairs-outsourcing market. The integration of artificial intelligence (AI) and data analytics into regulatory processes is streamlining compliance and submission procedures. In France, regulatory agencies are increasingly adopting digital tools to enhance efficiency and accuracy in regulatory submissions. This trend is likely to drive demand for outsourcing, as companies seek to leverage these technologies without investing heavily in-house capabilities. The healthcare regulatory-affairs-outsourcing market is projected to grow by 15% in 2025, as organizations recognize the potential of technology to improve regulatory compliance and reduce time-to-market for new products.





















Leave a Comment