Rising Prevalence of Porphyria
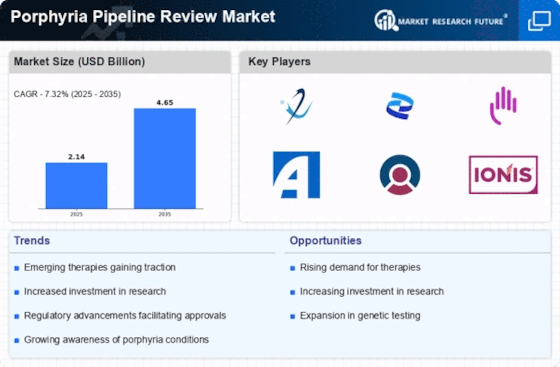
The increasing incidence of porphyria disorders is a pivotal driver for the Porphyria Pipeline Review Market. Recent estimates suggest that the prevalence of porphyria may be higher than previously recognized, with certain types affecting approximately 1 in 25,000 individuals. This rising prevalence necessitates the development of new therapies, thereby stimulating research and investment in the pipeline. As awareness grows among healthcare professionals and patients, the demand for effective treatments is likely to escalate, further propelling the market forward. The Porphyria Pipeline Review Market is thus positioned to benefit from this trend, as pharmaceutical companies seek to address the unmet needs of patients suffering from these rare conditions.
Advancements in Genetic Research
Advancements in genetic research are significantly influencing the Porphyria Pipeline Review Market. The identification of specific genetic mutations associated with various types of porphyria has opened new avenues for targeted therapies. For instance, gene therapy and personalized medicine approaches are being explored to provide tailored treatments for affected individuals. This shift towards precision medicine is expected to enhance treatment efficacy and safety, thereby attracting investment and interest from biopharmaceutical companies. As research continues to unveil the genetic underpinnings of porphyria, the Porphyria Pipeline Review Market is likely to experience a surge in innovative therapeutic options, catering to the diverse needs of patients.
Growing Patient Advocacy and Awareness
The role of patient advocacy groups in raising awareness about porphyria is increasingly significant within the Porphyria Pipeline Review Market. These organizations are instrumental in educating both the public and healthcare professionals about the challenges faced by individuals with porphyria. Their efforts have led to heightened visibility of the condition, which in turn drives demand for research and new treatment options. As advocacy groups collaborate with researchers and pharmaceutical companies, they help to shape the direction of the pipeline, ensuring that patient needs are prioritized. This growing movement is likely to foster a more robust Porphyria Pipeline Review Market, as stakeholders work together to address the complexities of these disorders.
Regulatory Incentives for Drug Development
Regulatory incentives play a crucial role in shaping the Porphyria Pipeline Review Market. Authorities are increasingly implementing policies that facilitate the development of treatments for rare diseases, including porphyria. Programs such as orphan drug designations and fast-track approvals are designed to expedite the review process for new therapies, encouraging pharmaceutical companies to invest in this area. These incentives not only reduce the time and cost associated with bringing new drugs to market but also enhance the attractiveness of the Porphyria Pipeline Review Market for investors. As a result, the landscape for porphyria treatments is likely to evolve rapidly, with a growing number of therapies entering the pipeline.
Increased Funding for Rare Disease Research
The Porphyria Pipeline Review Market is benefiting from increased funding directed towards rare disease research. Governments and private organizations are recognizing the importance of addressing rare conditions, leading to a rise in grants and financial support for research initiatives. In recent years, funding for rare diseases has seen a notable increase, with billions allocated to support clinical trials and drug development. This financial backing is crucial for advancing the pipeline of potential therapies for porphyria, as it enables researchers to explore novel treatment modalities. Consequently, the Porphyria Pipeline Review Market is poised for growth as more resources become available to tackle these challenging disorders.