Growing Demand for Gene Therapies
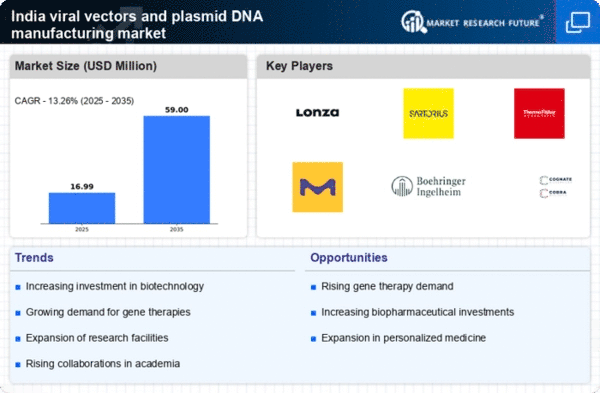
The rising prevalence of genetic disorders and chronic diseases in India is driving the demand for innovative treatment solutions. The viral vectors-and-plasmid-dna-manufacturing market is experiencing significant growth as gene therapies become more mainstream. According to recent estimates, the market for gene therapies in India is projected to reach approximately $1.5 billion by 2026, reflecting a compound annual growth rate (CAGR) of around 25%. This surge is largely attributed to advancements in genetic engineering and the increasing acceptance of gene therapies among healthcare professionals and patients. As a result, manufacturers are focusing on enhancing their production capabilities to meet the escalating demand, thereby propelling the growth of the viral vectors-and-plasmid-dna-manufacturing market in the country.
Supportive Government Initiatives
The Indian government is actively promoting biotechnology and biomanufacturing sectors, which is beneficial for the viral vectors-and-plasmid-dna-manufacturing market. Initiatives such as the Biotechnology Industry Research Assistance Council (BIRAC) and various funding programs are designed to support research and development in this field. These efforts are aimed at fostering innovation and facilitating the commercialization of biotechnological products. Additionally, the government is working to streamline regulatory processes, making it easier for companies to bring new therapies to market. This supportive environment is likely to encourage investment and growth in the viral vectors-and-plasmid-dna-manufacturing market, positioning India as a key player in the global biotechnology landscape.
Rising Collaborations and Partnerships
Collaborations between academic institutions, research organizations, and industry players are becoming increasingly common in India, significantly impacting the viral vectors-and-plasmid-dna-manufacturing market. These partnerships facilitate knowledge exchange and resource sharing, leading to accelerated research and development of new therapies. For instance, joint ventures between biotech firms and universities are enabling the development of innovative viral vectors and plasmid DNA technologies. This collaborative approach not only enhances the capabilities of manufacturers but also fosters a culture of innovation within the industry. As a result, the viral vectors-and-plasmid-dna-manufacturing market is likely to benefit from a more dynamic and interconnected ecosystem, driving growth and advancements in therapeutic solutions.
Increased Focus on Personalized Medicine
The trend towards personalized medicine is significantly influencing the viral vectors-and-plasmid-dna-manufacturing market. As healthcare providers increasingly recognize the importance of tailoring treatments to individual patient profiles, the demand for customized gene therapies is on the rise. This shift is prompting manufacturers to develop more versatile and adaptable production methods to cater to specific patient needs. The market for personalized medicine in India is expected to grow at a CAGR of around 20% over the next few years, indicating a robust opportunity for the viral vectors-and-plasmid-dna-manufacturing market. This focus on personalized approaches is likely to enhance patient outcomes and drive further innovation within the industry.
Technological Advancements in Manufacturing Processes
Technological innovations are playing a pivotal role in the evolution of the viral vectors-and-plasmid-dna-manufacturing market. The introduction of advanced manufacturing techniques, such as continuous bioprocessing and automated production systems, is enhancing efficiency and reducing costs. These advancements enable manufacturers to produce higher yields of viral vectors and plasmid DNA, which are essential for various therapeutic applications. Furthermore, the integration of artificial intelligence and machine learning in production processes is streamlining operations and improving quality control. As a result, the market is witnessing a shift towards more sophisticated manufacturing solutions, which is likely to attract further investment and drive growth in the viral vectors-and-plasmid-dna-manufacturing market.




















Leave a Comment