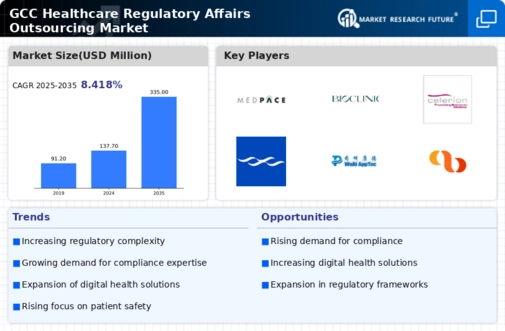
The GCC Healthcare Regulatory Affairs Outsourcing Market is characterized by a dynamic and rapidly evolving landscape that reflects the region's commitment to enhancing healthcare quality and compliance. As regulatory frameworks become increasingly complex, many healthcare enterprises seek to partner with outsourcing firms that specialize in navigating the multifaceted regulatory environments. Understanding the competitive landscape provides insights into how companies differentiate themselves through expertise, innovative solutions, and strategic alliances.
The growth of this market is driven by the need for streamlined regulatory processes, accelerated time-to-market for pharmaceutical and biotech products, and a heightened focus on patient safety and adherence to local regulations.
Competitors in this space are leveraging technology and skilled human resources to offer comprehensive services that cater to the diverse needs of companies seeking to penetrate the GCC healthcare market.Covance holds a significant presence within the GCC Healthcare Regulatory Affairs Outsourcing Market, known for its robust capabilities in regulatory consulting and compliance. The company has positioned itself as a leader by offering comprehensive regulatory services, including submission management, product lifecycle management, and regulatory strategy development tailored specifically for the GCC region.
Its strengths lie in its extensive experience working with local regulators, understanding regional legislative nuances, and its ability to provide tailored solutions that meet the unique demands of the market.
Covance also leverages its global reach and scientific expertise to deliver valuable insights that aid clients in navigating the complexities of healthcare regulations, thereby enhancing their operational efficiencies and expediting product approvals within the GCC markets.Medpace has also established a formidable presence in the GCC Healthcare Regulatory Affairs Outsourcing Market, offering several key services that include regulatory submissions, clinical trial management, and comprehensive advisory services tailored to local market requirements. Its strengths are underscored by a commitment to scientific integrity and a strategic approach to compliance in the rapidly evolving healthcare landscape of the GCC region.
Medpace's capability to foster partnerships with regulatory authorities enhances its effectiveness in facilitating clients' market entry and ensuring adherence to localized regulations. The company has also focused on expanding its footprint through strategic mergers and acquisitions, allowing it to bolster its service offerings and expertise in the GCC market. This proactive approach not only amplifies its product portfolio but also strengthens its position as a go-to provider for regulatory affairs outsourcing, helping clients efficiently navigate the complexities associated with bringing healthcare innovations to the GCC region.


















Leave a Comment