Globalization of Clinical Research
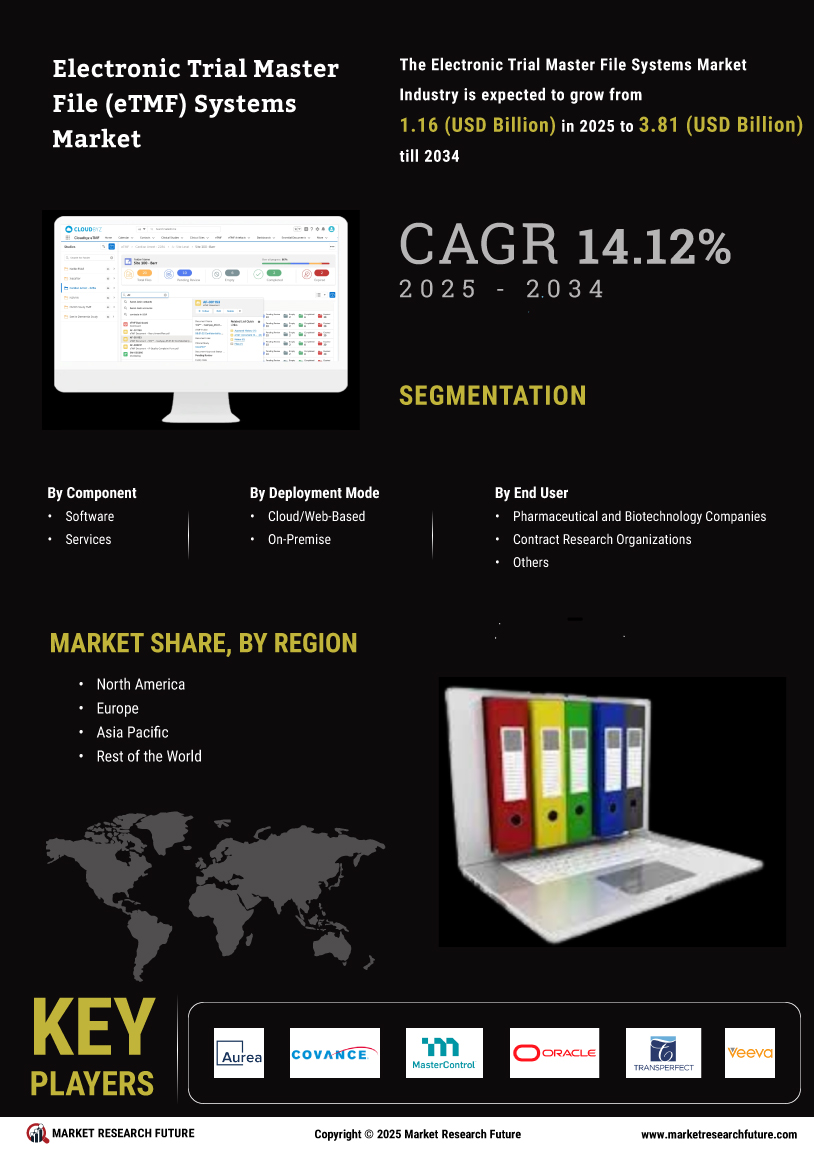
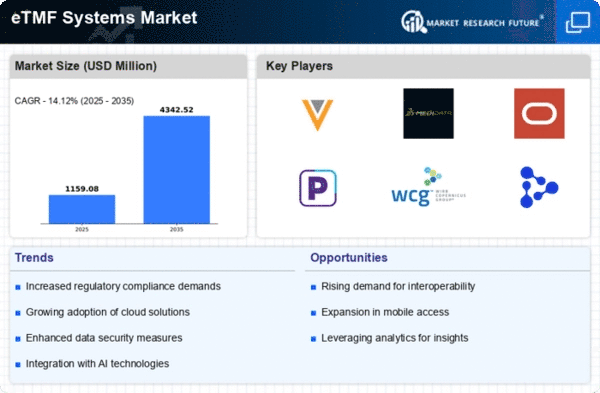
The globalization of clinical research significantly influences the Global Electronic Trial Master File (eTMF) Systems Market Industry. As pharmaceutical companies expand their operations across borders, the need for standardized documentation and data management becomes paramount. eTMF systems provide a centralized platform for managing trial documents from multiple regions, ensuring consistency and compliance with diverse regulatory requirements. This globalization trend not only enhances collaboration among international research teams but also accelerates the drug development process. The market's anticipated growth, from 1.02 USD Billion in 2024 to 4.35 USD Billion by 2035, underscores the critical role of eTMF systems in facilitating global clinical trials.
Increasing Demand for Clinical Trials
The Global Electronic Trial Master File (eTMF) Systems Market Industry experiences a surge in demand for clinical trials, driven by the need for efficient data management and regulatory compliance. As the number of clinical trials continues to rise, organizations are increasingly adopting eTMF systems to streamline processes and enhance collaboration among stakeholders. In 2024, the market is valued at 1.02 USD Billion, reflecting the growing recognition of eTMF systems as essential tools for managing trial documentation. This trend is expected to persist, with projections indicating a market growth to 4.35 USD Billion by 2035, highlighting the critical role of eTMF systems in the evolving landscape of clinical research.
Rising Focus on Patient-Centric Trials
The Global Electronic Trial Master File (eTMF) Systems Market Industry is witnessing a shift towards patient-centric clinical trials, which emphasizes the importance of patient engagement and experience. This trend is driven by the recognition that involving patients in the trial process can lead to better outcomes and higher retention rates. eTMF systems facilitate the management of patient-related data and documentation, enabling sponsors to tailor trials to meet patient needs effectively. As organizations increasingly prioritize patient-centric approaches, the demand for eTMF solutions is expected to rise, contributing to the market's growth. The projected CAGR of 14.09% from 2025 to 2035 indicates a robust future for eTMF systems in supporting this paradigm shift.
Regulatory Compliance and Standardization
Regulatory compliance remains a pivotal driver for the Global Electronic Trial Master File (eTMF) Systems Market Industry. As regulatory bodies impose stringent guidelines for clinical trial documentation, organizations are compelled to adopt eTMF solutions that ensure adherence to these standards. The integration of eTMF systems facilitates real-time access to trial data, thereby enhancing transparency and accountability. This compliance-driven approach not only mitigates risks associated with non-compliance but also fosters trust among stakeholders. The anticipated growth of the market, with a projected CAGR of 14.09% from 2025 to 2035, underscores the importance of regulatory alignment in the adoption of eTMF systems.
Technological Advancements in Data Management
Technological advancements play a crucial role in shaping the Global Electronic Trial Master File (eTMF) Systems Market Industry. Innovations in cloud computing, artificial intelligence, and data analytics are transforming how clinical trial data is managed and stored. These technologies enable organizations to enhance data integrity, streamline workflows, and improve overall efficiency. As eTMF systems evolve, they offer features such as automated document tracking and real-time collaboration, which are increasingly sought after by clinical trial sponsors. The market's growth trajectory, with an expected increase from 1.02 USD Billion in 2024 to 4.35 USD Billion by 2035, reflects the impact of these technological advancements on the industry.