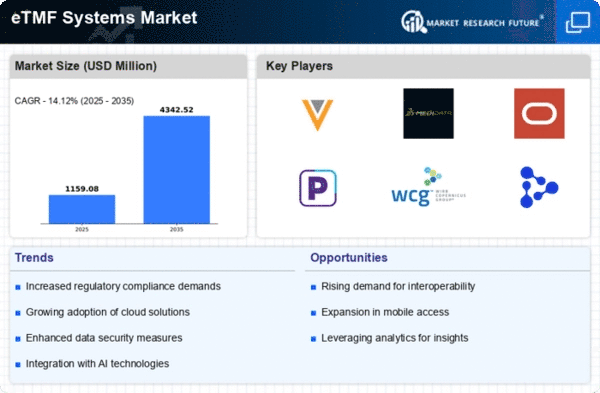
Top Industry Leaders in the eTMF Systems Market

Latest Electronic Trial Master File Systems Companies Update:
Veeva Systems Acquires Medidata in December 2023: This major acquisition consolidates two leading eTMF providers, potentially shaping the market landscape with combined offerings and expanded global reach.
eClinical Solutions Partners with IQVIA in November 2023: This collaboration aims to integrate eTMF solutions with IQVIA's expertise in clinical research, streamlining workflows and data management for sponsors and CROs.
Aurea Announces New Cloud-Based eTMF Solution in October 2023: This launch caters to the growing demand for secure and accessible eTMF systems, emphasizing scalability and compliance features.
Increased Adoption of SaaS (Software-as-a-Service) Models: Companies like Mastercontrol and Transperfect are witnessing rising adoption of their cloud-based eTMF solutions due to flexibility and cost-effectiveness.
List of Electronic Trial Master File Systems Key companies in the market
- Aurea
- Covance Inc.
- Mastercontrol Inc.
- Oracle
- Transperfect
- Veeva Systems
- ePharmaSolutions
- Phlex
- SureClinical Inc.