Emphasis on Data Security and Privacy
Data security and privacy concerns are paramount in the etmf systems market, particularly in the GCC region. With the increasing digitization of clinical trial data, organizations are prioritizing systems that ensure robust data protection measures. Regulatory bodies are imposing stricter guidelines regarding data handling, which is likely to drive the demand for etmf systems that incorporate advanced security features. Companies are investing in solutions that not only comply with regulations but also safeguard sensitive information from breaches. This focus on data security is expected to shape the etmf systems market, as organizations seek to mitigate risks associated with data management.
Growing Investment in Clinical Research
Investment in clinical research is on the rise in the GCC, which is positively impacting the etmf systems market. Governments and private entities are increasingly funding research initiatives to foster innovation and improve healthcare outcomes. This influx of capital is expected to drive the adoption of advanced etmf systems, as organizations look to modernize their data management practices. The market is projected to reach a valuation of over $200 million by 2027, reflecting a robust growth trajectory. As clinical research expands, the need for efficient and compliant data management solutions will become even more pronounced, further propelling the etmf systems market.
Rising Demand for Data Management Solutions
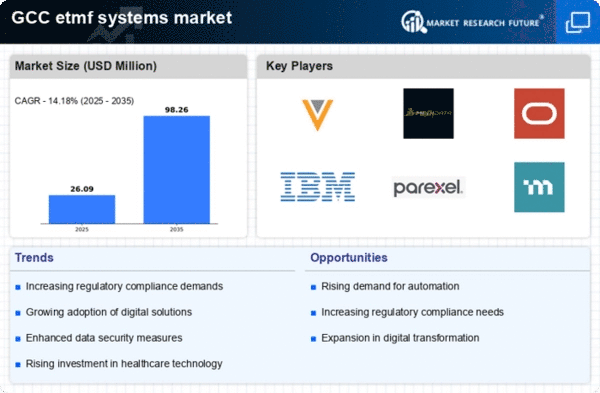
The etmf systems market is experiencing a notable increase in demand for efficient data management solutions. This trend is driven by the need for organizations to streamline their clinical trial processes and ensure compliance with regulatory standards. In the GCC region, the market for electronic trial master files is projected to grow at a CAGR of approximately 15% over the next five years. Companies are increasingly recognizing the importance of having a centralized system to manage trial data, which enhances data integrity and accessibility. As a result, the etmf systems market is likely to see a surge in adoption among pharmaceutical and biotechnology firms seeking to optimize their operations and improve overall efficiency.
Focus on Enhanced Collaboration Among Stakeholders
Collaboration among various stakeholders in clinical trials is becoming increasingly critical, thereby influencing the etmf systems market. The need for seamless communication between sponsors, clinical research organizations, and regulatory bodies is paramount. In the GCC, the emphasis on collaborative platforms is expected to drive market growth, as organizations seek to improve transparency and data sharing.. Enhanced collaboration can lead to reduced trial timelines and improved outcomes, which are essential for maintaining competitiveness in the industry. The etmf systems market is likely to benefit from this trend, as systems that facilitate real-time collaboration are in high demand.
Integration of Artificial Intelligence and Machine Learning
The integration of artificial intelligence (AI) and machine learning (ML) technologies is emerging as a transformative driver in the etmf systems market. These technologies offer the potential to enhance data analysis, automate processes, and improve decision-making in clinical trials. In the GCC, organizations are increasingly exploring AI-driven solutions to optimize their trial management processes. The ability to analyze vast amounts of data quickly and accurately can lead to more efficient trials and better patient outcomes. As AI and ML technologies continue to evolve, their incorporation into etmf systems is likely to become a key differentiator in the market.