Growing Focus on Patient-Centric Trials
The shift towards patient-centric clinical trials in China is emerging as a key driver for the etmf systems market. This approach emphasizes the importance of patient engagement and data collection from diverse populations, necessitating the use of advanced electronic systems to manage trial data effectively. As organizations strive to enhance patient experience and outcomes, the demand for flexible and scalable etmf solutions is likely to increase. Recent studies indicate that patient-centric trials can improve recruitment rates by up to 30%, highlighting the need for efficient data management systems. Consequently, the etmf systems market is expected to benefit from this trend, as companies seek to implement solutions that facilitate better patient interaction and data collection.
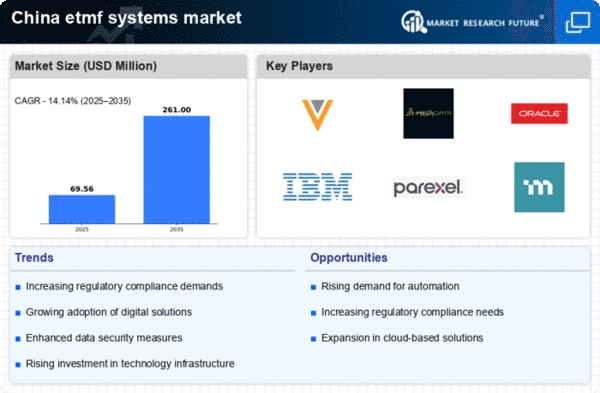
Rising Demand for Data Management Solutions
The increasing complexity of clinical trials in China has led to a rising demand for efficient data management solutions within the etmf systems market. As pharmaceutical and biotechnology companies seek to streamline their operations, the need for robust electronic trial master file systems becomes apparent. The market is projected to grow at a CAGR of approximately 15% over the next five years, driven by the necessity for accurate data collection and management. This trend is further supported by the growing number of clinical trials being conducted in China, which reached over 4,000 in 2025. Consequently, organizations are investing in etmf systems to enhance data integrity and compliance, ensuring that they meet both local and international regulatory standards.
Increased Investment in Research and Development
Investment in research and development (R&D) within the pharmaceutical sector in China is witnessing a notable increase, which is positively influencing the etmf systems market. With R&D spending projected to reach $60 billion by 2026, companies are prioritizing the adoption of electronic systems to enhance trial efficiency and data accuracy. This influx of capital is likely to drive the demand for advanced etmf solutions that can support the growing number of clinical trials. Furthermore, as organizations aim to bring innovative therapies to market faster, the need for streamlined data management processes becomes critical. Thus, the etmf systems market is positioned to grow in tandem with the expanding R&D landscape in China.
Government Initiatives to Enhance Clinical Research
The Chinese government has implemented various initiatives aimed at enhancing clinical research capabilities, which significantly impacts the etmf systems market. Policies promoting innovation and investment in healthcare technology are encouraging organizations to adopt advanced electronic systems for trial management. For instance, the government has allocated over $1 billion to support digital transformation in the healthcare sector, which includes the adoption of etmf systems. This financial backing is likely to accelerate the integration of technology in clinical trials, fostering a more efficient research environment. As a result, companies are increasingly recognizing the importance of adopting electronic systems to comply with regulatory requirements and improve operational efficiency.
Emergence of Artificial Intelligence in Clinical Trials
The integration of artificial intelligence (AI) technologies into clinical trials is becoming a transformative force within the etmf systems market. In China, AI applications are being utilized to optimize trial design, patient recruitment, and data analysis, thereby enhancing overall trial efficiency. As organizations increasingly recognize the potential of AI to reduce costs and improve outcomes, the demand for etmf systems that incorporate these technologies is likely to rise. Reports suggest that AI can reduce trial timelines by up to 20%, making it an attractive option for companies looking to expedite their research processes. Consequently, the etmf systems market is expected to evolve, with a growing emphasis on AI-driven solutions that facilitate smarter data management.