Consumer Demand for Accessibility
Consumer demand for accessibility is a driving force in the COVID Testing Kit Market. As awareness of COVID-19 persists, individuals seek convenient and reliable testing options. The rise in demand for over-the-counter testing kits reflects a shift towards self-management of health. Market data indicates that sales of at-home testing kits have surged, with many consumers preferring the ease of testing in their own environments. This trend is particularly pronounced in regions where healthcare access is limited. The COVID Testing Kit Market is responding to this demand by expanding distribution channels and enhancing product availability. As consumers continue to prioritize accessibility, manufacturers are likely to innovate further, ensuring that testing solutions meet diverse needs.
Regulatory Support and Guidelines
Regulatory support plays a crucial role in shaping the COVID Testing Kit Market. Governments and health organizations worldwide have established guidelines to ensure the safety and efficacy of testing kits. This regulatory framework facilitates the approval process for new testing technologies, thereby accelerating their entry into the market. For instance, emergency use authorizations have enabled rapid deployment of various testing kits, which has been essential in managing public health crises. The presence of clear regulations not only instills confidence among consumers but also encourages manufacturers to innovate. As regulatory bodies continue to adapt to emerging challenges, the COVID Testing Kit Market is expected to benefit from streamlined processes that promote the development of reliable testing solutions.
Technological Advancements in Testing
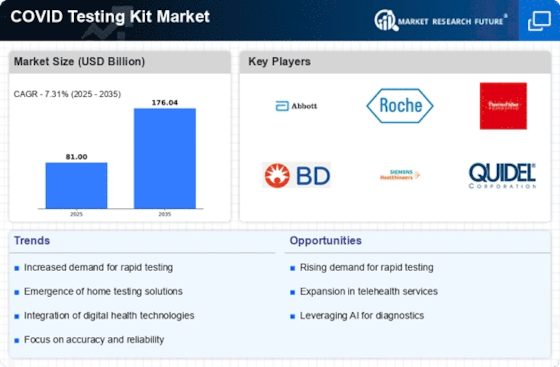
The COVID Testing Kit Market is experiencing rapid technological advancements that enhance testing accuracy and speed. Innovations such as PCR and antigen testing have revolutionized the way COVID-19 is diagnosed. The introduction of at-home testing kits has further expanded accessibility, allowing individuals to test without visiting healthcare facilities. According to recent data, the market for at-home testing kits is projected to grow significantly, driven by consumer preference for convenience and privacy. These advancements not only improve the user experience but also contribute to more efficient public health responses. As technology continues to evolve, the COVID Testing Kit Market is likely to see the emergence of even more sophisticated testing solutions, potentially increasing market penetration and consumer trust.
Emerging Variants and Continued Testing Needs
The emergence of new COVID-19 variants is a persistent driver in the COVID Testing Kit Market. As variants evolve, the need for effective testing solutions remains critical to controlling outbreaks. Public health authorities emphasize the importance of ongoing testing to identify and respond to these variants swiftly. Market analysis indicates that the demand for versatile testing kits capable of detecting multiple strains is on the rise. This ongoing need for adaptability in testing solutions suggests that the COVID Testing Kit Market will continue to evolve, with manufacturers focusing on developing kits that can address the challenges posed by emerging variants.
Increased Investment in Healthcare Infrastructure
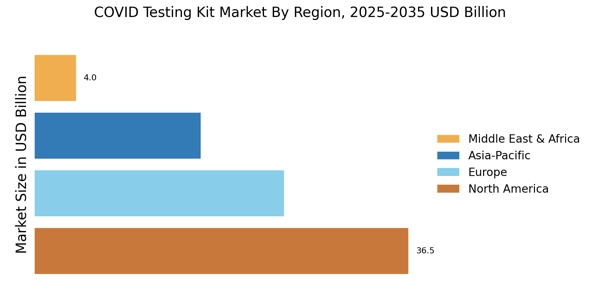
Increased investment in healthcare infrastructure is significantly impacting the COVID Testing Kit Market. Governments and private entities are channeling resources into enhancing laboratory capabilities and expanding testing facilities. This investment is crucial for improving the overall efficiency of testing processes and ensuring that adequate resources are available to meet public health demands. Data suggests that countries with robust healthcare systems are better equipped to handle testing surges, leading to a more resilient market. As investments continue to flow into healthcare, the COVID Testing Kit Market is poised for growth, with improved infrastructure facilitating faster and more widespread testing solutions.