Emergence of New Variants
The emergence of new variants continues to influence the dynamics of the covid testing-kit market. As variants evolve, the need for effective testing solutions that can accurately detect these strains becomes paramount. In 2025, the market sees a shift towards tests that are specifically designed to identify multiple variants, ensuring that public health measures remain effective. The covid testing-kit market is responding by investing in research and development to create more sophisticated testing technologies. This focus on variant detection is likely to drive sales, as healthcare providers and consumers seek reliable testing options. Moreover, the ongoing threat of new variants may lead to increased public awareness and demand for regular testing, further propelling market growth.
Government Initiatives and Funding
Government initiatives play a crucial role in shaping the covid testing-kit market. In 2025, federal and state governments have allocated substantial funding to enhance testing infrastructure and accessibility. This financial support is aimed at increasing the availability of testing kits, particularly in underserved communities. The covid testing-kit market benefits from these initiatives, as they facilitate partnerships between public health agencies and private manufacturers, leading to the development of affordable testing solutions. Additionally, government campaigns promoting regular testing have further stimulated market growth, with an estimated increase of 30% in testing kit distribution across various states. Such efforts underscore the importance of proactive testing in managing public health and controlling potential outbreaks.
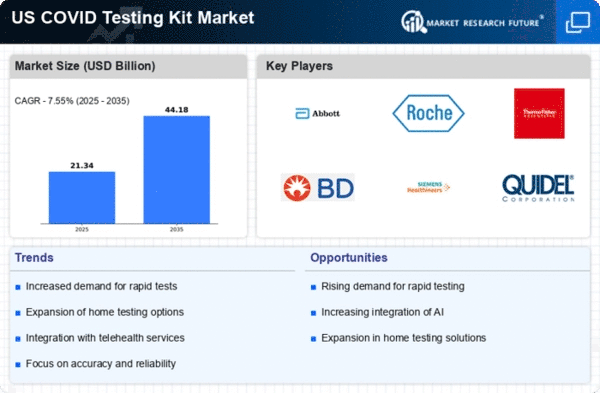
Increased Demand for Rapid Testing
The covid testing-kit market experiences heightened demand for rapid testing solutions, driven by the need for quick results in various settings, including workplaces and schools. As organizations prioritize safety, the market for rapid antigen tests has surged, with sales reaching approximately $2 billion in 2025 alone. This trend indicates a shift towards immediate testing capabilities, allowing for timely decision-making and minimizing the spread of infections. The covid testing-kit market is adapting to this demand by innovating and expanding product lines to include more rapid testing options, which are essential for effective public health responses. Furthermore, the convenience of rapid tests is appealing to consumers, as they can be administered at home or in non-clinical environments, thus enhancing accessibility and encouraging widespread testing.
Integration of Digital Health Solutions
The integration of digital health solutions into the covid testing-kit market is transforming how testing is conducted and managed. In 2025, many testing kits are now accompanied by mobile applications that facilitate result tracking and reporting. This technological advancement enhances user experience and encourages more individuals to participate in regular testing. The covid testing-kit market is witnessing a trend where digital platforms not only provide results but also offer guidance on next steps, such as isolation or vaccination. This integration is likely to improve overall public health outcomes by ensuring that individuals receive timely information. Furthermore, the data collected through these digital solutions can assist health authorities in monitoring trends and making informed decisions.
Rising Consumer Awareness and Education
Rising consumer awareness regarding the importance of testing is significantly impacting the covid testing-kit market. In 2025, educational campaigns aimed at informing the public about the benefits of regular testing have gained traction. This increased awareness is likely to lead to higher demand for testing kits, as individuals recognize their role in preventing the spread of infections. The covid testing-kit market is responding by enhancing marketing strategies to reach a broader audience, emphasizing the ease of use and accessibility of testing solutions. As consumers become more informed, they are more inclined to purchase testing kits for personal use, contributing to market growth. This trend suggests a shift towards a more proactive approach to health management among the general population.