Top Industry Leaders in the COVID Testing Kit Market
 *Disclaimer: List of key companies in no particular orderLatest COVID-19 testing kits Companies Update
*Disclaimer: List of key companies in no particular orderLatest COVID-19 testing kits Companies Update
-
February 2023: NanoSpot.ai has recently introduced the SARS-CoV-2 Total Antibody Test in the European market. The semi-quantitative antibody test is conducted utilizing an agglutination-based methodology, alongside an artificial intelligence (AI)-enabled smartphone application, within the point-of-care setting. In May of this year, the business successfully obtained certification for the device, allowing its usage in the European Union and other nations that acknowledge the CE Mark. The complete procedure, encompassing sample collection to result acquisition, is accomplished within a time frame of under three minutes and does not necessitate the use of a complex diagnostic apparatus. The test has demonstrated a sensitivity of 97.6% and a specificity of 100%, making it suitable for use in both home and professional settings.
-
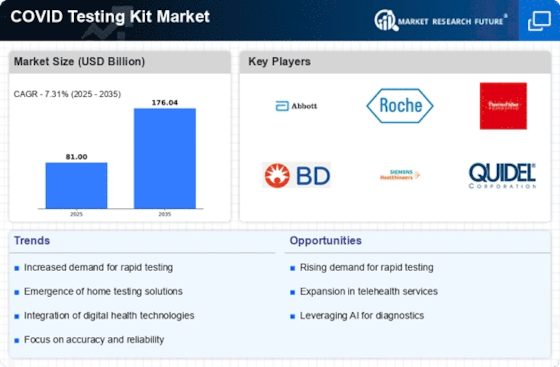
April 2023: Thermo Fisher Scientific unveiled the initial set of 37 real-time PCR test kits, all of which have received the CE-IVD marking. The aforementioned kits are designed for utilization in conjunction with the QuantStudio Dx equipment series offered by the corporation. They are expected to be made accessible to clients at a later point during the current year. Thermo Fisher showcased test kits designed for the screening of human papillomavirus and herpes simplex virus using their QuantStudio 5 Dx (QS5 Dx) real-time PCR system at the European Congress of Clinical Microbiology and Infectious Diseases. Thermo Fisher is scheduled to introduce supplementary diagnostic tests for sexually transmitted and blood-borne illnesses, such as chlamydia, gonorrhea, and HIV, during the initial six months of 2023. Subsequently, the company plans to launch assays targeting respiratory tract infections, infections occurring in transplant and immunocompromised individuals, as well as hepatitis.List of COVID-19 testing kits Key companies in the market - Abbott (US)
- Hoffmann-La Roche AG (Switzerland)
- Danaher Corporation (US)
- Bio-Rad Laboratories, Inc. (US)
- Thermo Fisher Scientific Inc. (US)
- Qiagen (Germany)
- BioMérieux SA (France)
- Siemens AG (Germany)
- GenMark (US)
- BGI Group (China)