North America : Market Leader in Innovation
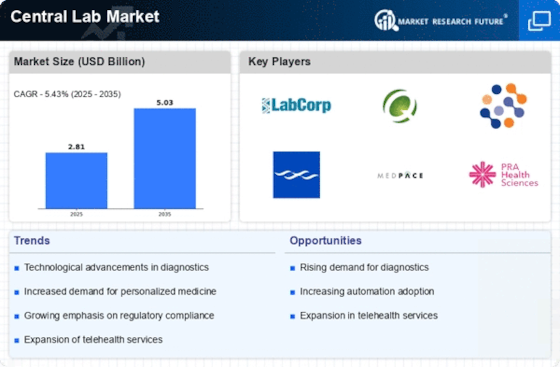
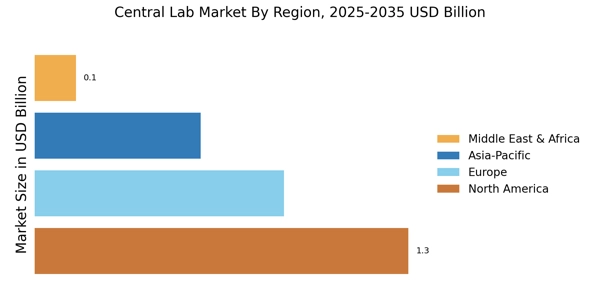
North America is the largest market for central lab services, holding approximately 45% of the global market share. The region benefits from advanced healthcare infrastructure, high demand for diagnostic services, and significant investments in research and development. Regulatory support from agencies like the FDA further catalyzes growth, ensuring compliance and innovation in laboratory practices. The United States is the primary driver, with key players such as LabCorp and Quest Diagnostics leading the market. The competitive landscape is characterized by a mix of established firms and emerging players, all vying for market share. The presence of major companies like Charles River Laboratories and Medpace enhances the region's reputation as a hub for laboratory services, fostering innovation and collaboration across the sector.
Europe : Emerging Hub for Diagnostics
Europe is witnessing significant growth in the central lab market, accounting for approximately 30% of the global share. The region's growth is driven by increasing healthcare expenditure, a rising prevalence of chronic diseases, and a strong emphasis on research and development. Regulatory frameworks, such as the EU central labs in clinical trials Regulation, are enhancing the operational landscape for laboratories, promoting efficiency and compliance.
Leading countries include Germany, France, and the UK, with companies like Eurofins Scientific and Synlab playing pivotal roles. The competitive landscape is robust, with a mix of local and international players. The presence of advanced technologies and a skilled workforce further strengthens Europe's position in the central lab market, making it a key player in the global arena.
Asia-Pacific : Rapidly Growing Market Potential
Asia-Pacific is emerging as a significant player in the central lab market, holding around 20% of the global market share. The region's growth is fueled by increasing healthcare investments, a rising population, and a growing demand for advanced diagnostic services. Countries like China and India are witnessing rapid advancements in healthcare infrastructure, supported by government initiatives and favorable regulations that encourage laboratory development. China is the largest market in the region, with Wuxi AppTec leading the charge. The competitive landscape is evolving, with both local and international players expanding their presence. The region's focus on innovation and technology adoption is driving the growth of central lab services, positioning Asia-Pacific as a future leader in the global market.
Middle East and Africa : Emerging Market with Challenges
The Middle East and Africa region is gradually developing its central lab market, currently holding about 5% of the global share. Growth is driven by increasing healthcare investments, a rising prevalence of diseases, and government initiatives aimed at improving healthcare infrastructure. However, challenges such as regulatory hurdles and varying levels of healthcare access remain significant barriers to rapid growth. Countries like South Africa and the UAE are at the forefront of this development, with local players and international firms exploring opportunities. The competitive landscape is characterized by a mix of established companies and new entrants, all aiming to enhance laboratory services. The region's potential for growth is substantial, particularly as healthcare systems continue to evolve and improve.