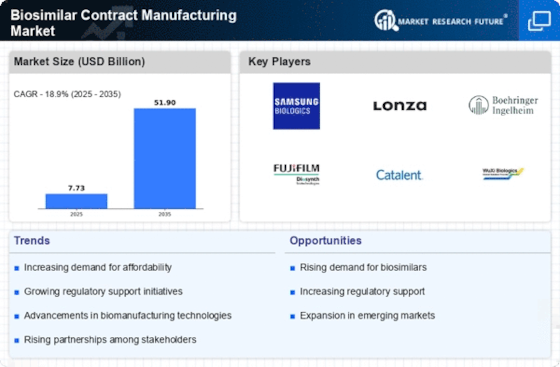
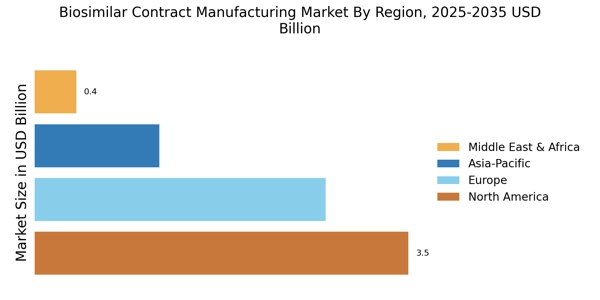
Cost-Effectiveness of Biosimilars
The cost-effectiveness of biosimilars compared to their reference biologics is a significant driver for the Biosimilar Contract Manufacturing Market. As healthcare costs continue to rise, payers and patients are increasingly seeking more affordable treatment options. Biosimilars offer a viable solution, often priced 20-30% lower than their reference products. This price differential not only makes biosimilars more accessible to patients but also encourages healthcare providers to prescribe them. Consequently, pharmaceutical companies are motivated to engage in contract manufacturing to produce biosimilars, thereby expanding their portfolios and meeting market demand. The Biosimilar Contract Manufacturing Market is thus likely to thrive as the emphasis on cost-effective healthcare solutions intensifies.
Regulatory Support and Frameworks
The establishment of supportive regulatory frameworks for biosimilars is a crucial factor influencing the Biosimilar Contract Manufacturing Market. Regulatory agencies are increasingly recognizing the importance of biosimilars in enhancing patient access to essential medications. For instance, streamlined approval processes and guidelines for biosimilar development have been implemented in various regions, facilitating faster market entry. This regulatory support not only encourages pharmaceutical companies to explore biosimilar options but also enhances the confidence of contract manufacturers in investing in biosimilar production capabilities. As a result, the Biosimilar Contract Manufacturing Market is likely to experience accelerated growth, driven by the favorable regulatory landscape that promotes the development and commercialization of biosimilars.
Growing Focus on Personalized Medicine
The increasing emphasis on personalized medicine is shaping the Biosimilar Contract Manufacturing Market. As healthcare shifts towards tailored treatment approaches, the demand for biosimilars that can be customized to meet individual patient needs is rising. This trend is prompting pharmaceutical companies to explore biosimilar options that align with personalized treatment paradigms. Additionally, the integration of advanced analytics and data-driven insights into biosimilar development is enhancing the ability to create targeted therapies. Consequently, contract manufacturers are likely to adapt their production capabilities to accommodate the growing demand for personalized biosimilars, thereby contributing to the expansion of the Biosimilar Contract Manufacturing Market.
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases such as diabetes, cancer, and autoimmune disorders is a primary driver for the Biosimilar Contract Manufacturing Market. As these conditions require long-term treatment, the demand for biologics, including biosimilars, is expected to surge. According to recent estimates, the prevalence of chronic diseases is projected to increase significantly, leading to a heightened need for affordable treatment options. This trend is likely to encourage pharmaceutical companies to invest in biosimilar development and contract manufacturing, as they seek to provide cost-effective alternatives to expensive biologic therapies. Consequently, the Biosimilar Contract Manufacturing Market is poised for growth as manufacturers align their capabilities to meet the increasing demand for biosimilars that address chronic health issues.
Technological Advancements in Manufacturing Processes
Technological advancements in biomanufacturing processes are transforming the Biosimilar Contract Manufacturing Market. Innovations such as single-use technologies, continuous manufacturing, and improved cell culture techniques are enhancing production efficiency and reducing costs. These advancements enable contract manufacturers to produce biosimilars at a larger scale while maintaining quality and compliance with regulatory standards. As technology continues to evolve, it is expected that the capabilities of contract manufacturers will expand, allowing for the development of a wider range of biosimilars. This trend is likely to attract more pharmaceutical companies to partner with contract manufacturers, further propelling the growth of the Biosimilar Contract Manufacturing Market.