Market Trends
Key Emerging Trends in the Biosimilar Contract Manufacturing Market
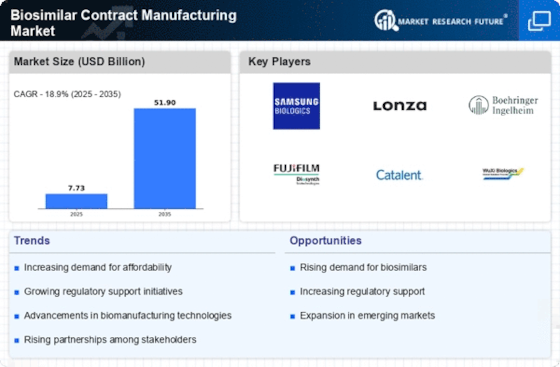
This Biosimilar Contract Manufacturing Market is experiencing remarkable patterns that show the changing nature of biopharmaceutical manufacturing and the increasing requirement for cost-effective alternatives to conventional biologics. Adoption of biosimilars as a viable way of cutting down on healthcare costs is one of such important trends. Biosimilars, which are essentially similar to authorized biologics, offer a better value alternative with similar safety and efficacy profiles. This is consistent with other efforts in the sector to improve access to bio pharmaceuticals as well as address economic challenges related to high price tags placed on biologics.
Market trends within the Biosimilar Contract Manufacturing Market are shaped by technological advancements. The progress made in terms of bioprocessing technologies, analytical techniques and cell line development has increased biosimilar manufacturing efficiency and scalability. By doing this, contract manufacturing organizations (CMOs) have been able to provide all- encompassing services at affordable prices, ranging from cell-line development through commercial scale production, thus leading to overall growth in the biosimilar market.
Moreover, there has been an increase in outsourcing biosimilar manufacture by firms to specialized CMOs. Biotech companies are increasingly dependent upon contract manufacturers due their expertise, infrastructure and flexibility in capacities for manufacturing. While CMOs take care of complexities involved in producing biosimilars; companies outsource so as concentrate on research development and commercialization. This reflects a strategy geared towards streamlining operations and reducing time-to-market for their own products.
Another key trend is the global expansion of biosimilar contract manufacturing services. Even though many countries have shown interest in its manufacturing, proximity to key markets remains important while regulatory compliance becomes easy when these organizations enter diverse locations. The globalization process also leads into efficient supply chain management that comes with improved accessibility across borders.
Regulatory developments and standards within the biopharmaceutical industry also influence the Biosimilar Contract Manufacturing Market. To ensure appropriate quality, safety and effectiveness of biosimilars, regulatory agencies are setting guidelines and frameworks. In response to this changing regulatory environment, CMOs have had to improve on their capabilities so as to comply with the requirements and provide manufacturing services that align with global regulatory needs. This reflects the importance of regulatory compliance in biosimilar manufacturing and its impact on market dynamics.
Moreover, market trends are influenced by increasing collaborations and partnerships between developers of biosimilars and contract manufacturers. Through these alliances, firms can take advantage of their research expertise, development strength, knowledge through mutual sharing, and creation together with other organizations whose specialization is manufacturing to come up with a more robust competitive offer. Overall advancement in technology for producing biosimilars is also boosted by collaborations.
This Biosimilar Contract Manufacturing Market has seen a major trending towards emphasis on sustainability and environmental responsibility. Green practices have been put into place whereby CMOs are concerned about resource optimization as well as deployment of environmentally friendly technologies that help in reducing ecological footprint in biopharmaceutical production. This trend is consistent with the broader industry’s commitment to sustainable practices and corporate social responsibility.

















Leave a Comment