Market Analysis
In-depth Analysis of Biosimilar Contract Manufacturing Market Industry Landscape
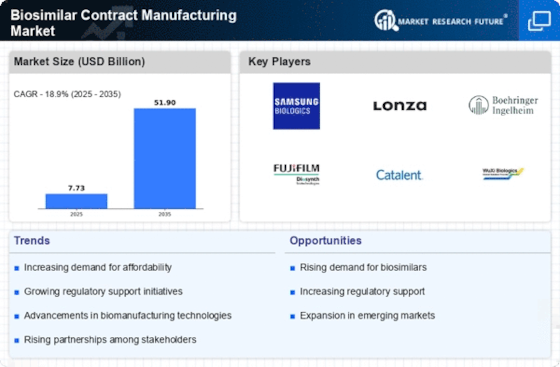
The growth and dynamics of the Biosimilar Contract Manufacturing market are determined by multiple factors that act in combination. The emergence of biosimilars, which are highly similar but not identical copies of existing biologic drugs, as cheaper alternatives to branded biologics has attracted attention. The market for biosimilar contract manufacturing is recognized through its drivers, challenges, trends and opportunities.
One major driver pushing the Biosimilar Contract Manufacturing market forward is the need for affordable biologic therapies. This alternative is much cheaper for both patients and healthcare systems when compared to their original product thus promoting their use. High cases of chronic diseases coupled with expiry of patents on biological drugs have created an enabling environment for the development and manufacturing of biosimilars thereby calling for contract manufacturing services.
Market dynamics are heavily influenced by advances in bioprocessing and manufacturing technologies. Such advanced manufacturing capabilities needed by biologics demand a more sophisticated approach since they are complex, with CMCs providing expertise and infrastructure. Continuous improvements within cell culture techniques, purification processes, and analytical methods improve productivity and quality in biosimilar production. Thus, CMOs that invest in cutting-edge facilities as well as technologies can be better at meeting regulatory conditions associated with biosimilar manufacturing.
Nevertheless, there is difficulty in the Biosimilar Contract Manufacturing market especially due to regulatory complexities. The approval pathway for biosimilars involves rigorous evaluations aimed at confirming similarity in respectively safety and quality terms. Both developers of these products as well as their contract manufacturers face problems navigating through differing regional demands hence keeping pace with global regulations. Also, intellectual property issues together with legal considerations might pose roadblocks during development or manufacture phase of biosimilars.
Another trend observed within this segment is globalization of services for biosimilar production around the world. For example, increased need for these products globally means that CMOs establish themselves in different locations across the globe. This tendency comes from diversifying into available markets following regional specifications for manufacturing purposes and other factors that drive the demand for biosimilars in different healthcare systems.
Similarly, market dynamics also indicate that strategic collaboration and partnerships are vital in the biosimilar ecosystem. In order to leverage on their expertise and infrastructure of contract manufacturing organizations (CMOs), biosimilar developers frequently engage them in collaborations thereby concentrating on research, development and commercialization issues. These alliances make it possible to simplify the whole process of developing biosimilars as well as boost these products’ effectiveness compared to those of competitors within the market.
From a geographical perspective, the Biosimilar Contract Manufacturing market has diverse characteristics. Some key markets for pharmaceuticals and biotechnology such as North America and Europe are currently experiencing rapid growth. However, emerging markets like Asia-Pacific have become more attractive due to lower-cost manufacturing capabilities, improving bioprocessing know-how and a rising demand for bio-similar therapies.

















Leave a Comment