Recombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Mammalian
Non-Mammalian
Oncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
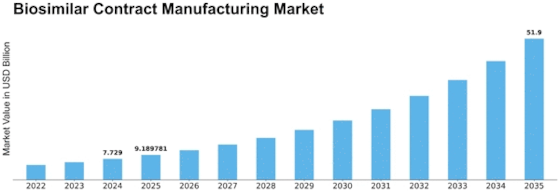
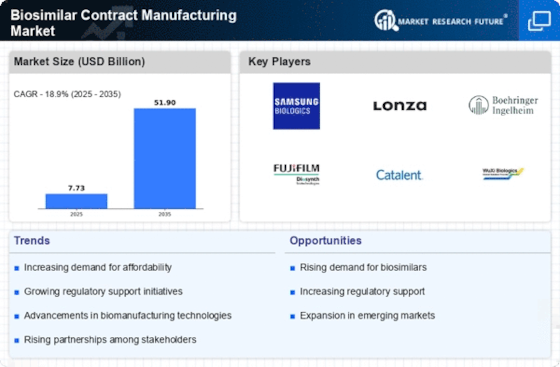
Biosimilar Contract Manufacturing Regional Outlook (USD Billion, 2018-2032)
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Germany Outlook (USD Billion, 2018-2032)
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others
Biosimilar Contract Manufacturing by ProductRecombinant Non-glycosylated Proteins
Recombinant Glycosylated Proteins
Biosimilar Contract Manufacturing by Production TechnologyMammalian
Non-Mammalian
Biosimilar Contract Manufacturing by ApplicationOncology
Blood Disorders
Growth Hormonal Deficiency
Chronic & Autoimmune Disorders
Rheumatoid Arthritis
Others

















Leave a Comment