Emergence of Personalized Medicine
The rise of personalized medicine is significantly influencing the Pharmaceutical Contract Manufacturing Organization Market CMO Market. As healthcare shifts towards tailored therapies, the demand for manufacturing processes that can accommodate individualized treatments is increasing. This trend necessitates CMOs to adapt their capabilities to produce small batches of customized drugs efficiently. Market analysis suggests that the personalized medicine sector is expected to expand rapidly, with a projected growth rate of over 15% annually. This evolution presents both challenges and opportunities for CMOs, as they must innovate to meet the unique demands of personalized therapies while ensuring cost-effectiveness and scalability.
Expansion of Outsourcing Practices
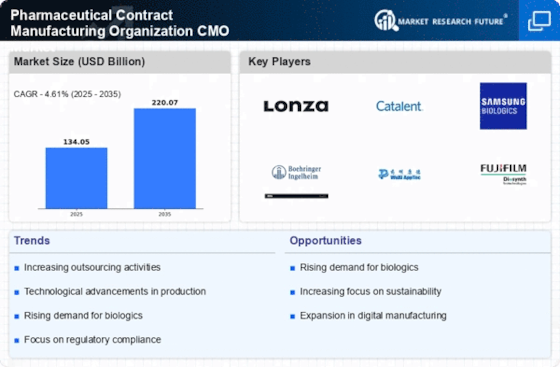
The trend of outsourcing within the Pharmaceutical Contract Manufacturing Organization Market CMO Market continues to gain momentum. Pharmaceutical companies are increasingly recognizing the strategic advantages of partnering with CMOs to streamline operations and reduce costs. By outsourcing manufacturing processes, companies can focus on core competencies such as research and development. Recent data indicates that the outsourcing market in pharmaceuticals is projected to reach approximately USD 200 billion by 2026. This shift not only allows for greater flexibility and scalability but also enables companies to leverage the specialized expertise of CMOs, thereby enhancing overall efficiency and productivity in drug manufacturing.
Technological Integration and Automation
Technological integration and automation are transforming the Pharmaceutical Contract Manufacturing Organization Market CMO Market. The adoption of advanced manufacturing technologies, such as robotics and artificial intelligence, is enhancing operational efficiency and reducing production costs. CMOs are increasingly investing in automation to streamline processes, improve accuracy, and minimize human error. Reports indicate that the market for automated pharmaceutical manufacturing is anticipated to grow significantly, driven by the need for faster production cycles and higher quality standards. This technological evolution not only benefits CMOs but also pharmaceutical companies, as it enables them to bring products to market more rapidly and efficiently.
Increasing Complexity of Drug Formulations
The Pharmaceutical Contract Manufacturing Organization Market CMO Market is witnessing a notable rise in the complexity of drug formulations. As pharmaceutical companies strive to develop more effective therapies, the demand for specialized manufacturing capabilities has surged. This complexity often necessitates advanced technologies and expertise, which CMOs are well-positioned to provide. According to industry reports, the market for complex generics and biologics is expected to grow significantly, with projections indicating a compound annual growth rate of over 10% in the coming years. This trend suggests that CMOs will play a crucial role in supporting pharmaceutical companies in navigating the intricacies of modern drug development, thereby enhancing their market presence.
Regulatory Compliance and Quality Assurance
Regulatory compliance remains a pivotal driver in the Pharmaceutical Contract Manufacturing Organization Market CMO Market. As regulatory bodies impose stringent guidelines to ensure drug safety and efficacy, CMOs are increasingly tasked with maintaining high standards of quality assurance. This necessity for compliance has led to the adoption of advanced quality management systems and practices within CMOs. The market for quality assurance services in pharmaceuticals is expected to grow, reflecting the increasing importance of regulatory adherence. Consequently, CMOs that prioritize compliance and quality are likely to gain a competitive edge, as pharmaceutical companies seek reliable partners to navigate the complex regulatory landscape.