Increasing Demand for Biologics
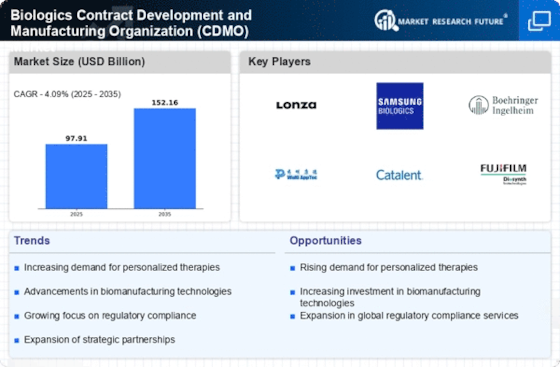
The Biologics Contract Development and Manufacturing Organization Market (CDMO) Market is experiencing a notable surge in demand for biologics, driven by the rising prevalence of chronic diseases and the aging population. As more pharmaceutical companies pivot towards biologics, the need for specialized manufacturing capabilities becomes paramount. According to recent data, the biologics market is projected to reach approximately USD 500 billion by 2026, indicating a robust growth trajectory. This trend compels CDMOs to enhance their production capacities and invest in advanced technologies to meet the escalating demand. Furthermore, the shift towards personalized medicine necessitates flexible manufacturing solutions, which CDMOs are well-positioned to provide. Consequently, the increasing demand for biologics is a significant driver for the Biologics Contract Development and Manufacturing Organization Market (CDMO) Market.
Rising Investment in Biopharmaceuticals
The Biologics Contract Development and Manufacturing Organization Market (CDMO) Market is witnessing a surge in investment in biopharmaceuticals, which is significantly influencing market dynamics. Venture capital funding and mergers and acquisitions are on the rise, as investors recognize the potential of biologics in addressing unmet medical needs. In 2025, investments in biopharmaceuticals are expected to exceed USD 300 billion, reflecting a strong commitment to research and development. This influx of capital is likely to enhance the capabilities of CDMOs, enabling them to expand their service offerings and improve production technologies. As biopharmaceutical companies seek to outsource manufacturing to focus on core competencies, the demand for CDMO services is expected to grow, further propelling the Biologics Contract Development and Manufacturing Organization Market (CDMO) Market.
Focus on Cost-Effectiveness and Efficiency
Cost-effectiveness is a pivotal driver in the Biologics Contract Development and Manufacturing Organization Market (CDMO) Market. As pharmaceutical companies strive to reduce operational costs while maintaining high-quality standards, outsourcing to CDMOs has become an attractive option. CDMOs offer specialized expertise and economies of scale that can lead to significant cost savings for their clients. In 2025, it is anticipated that the cost of biologics manufacturing will decrease by approximately 15% due to advancements in production techniques and increased competition among CDMOs. This trend encourages more companies to consider outsourcing as a viable strategy to enhance efficiency and focus on innovation. Consequently, the emphasis on cost-effectiveness is likely to drive growth within the Biologics Contract Development and Manufacturing Organization Market (CDMO) Market.
Technological Innovations in Manufacturing
Technological advancements are reshaping the landscape of the Biologics Contract Development and Manufacturing Organization Market (CDMO) Market. Innovations such as continuous manufacturing, single-use technologies, and automation are enhancing production efficiency and reducing costs. For instance, the adoption of single-use bioreactors has streamlined processes, allowing for quicker turnaround times and reduced contamination risks. As a result, CDMOs are increasingly able to offer competitive pricing and faster delivery to their clients. Moreover, the integration of data analytics and artificial intelligence in manufacturing processes is enabling CDMOs to optimize operations and improve product quality. This technological evolution is not only attracting new clients but also fostering partnerships between CDMOs and biotech firms, thereby driving growth in the Biologics Contract Development and Manufacturing Organization Market (CDMO) Market.
Regulatory Compliance and Quality Assurance
Regulatory compliance remains a critical driver within the Biologics Contract Development and Manufacturing Organization Market (CDMO) Market. As the biologics sector is heavily regulated, CDMOs must adhere to stringent guidelines set forth by regulatory bodies. This compliance ensures that products meet safety and efficacy standards, which is essential for gaining market approval. The complexity of biologics manufacturing, including the need for rigorous quality assurance processes, further emphasizes the importance of regulatory adherence. CDMOs that excel in maintaining compliance not only enhance their reputation but also attract more clients seeking reliable partners. The increasing focus on quality assurance is likely to drive investments in advanced quality control technologies, thereby fostering growth within the Biologics Contract Development and Manufacturing Organization Market (CDMO) Market.