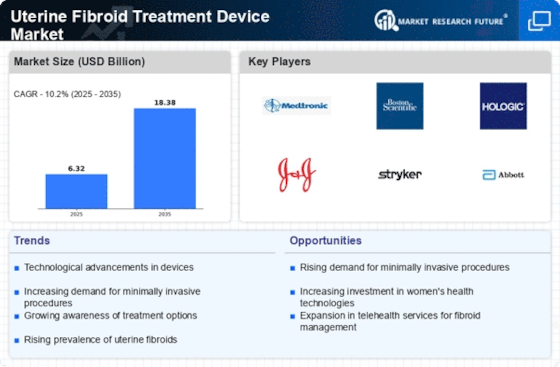
Rising Prevalence of Uterine Fibroids
The increasing incidence of uterine fibroids is a primary driver for the Uterine Fibroid Treatment Device Market. Studies indicate that approximately 70 to 80 percent of women develop fibroids by the age of 50. This rising prevalence necessitates effective treatment options, thereby propelling the demand for innovative treatment devices. As awareness about uterine fibroids grows, more women seek medical intervention, which further stimulates market growth. The Uterine Fibroid Treatment Device Market is likely to expand as healthcare providers adopt advanced technologies to address this widespread condition. Furthermore, the growing emphasis on women's health issues has led to increased funding for research and development in this area, potentially enhancing the availability of effective treatment devices.
Increasing Investment in Women's Health
Investment in women's health is a significant driver for the Uterine Fibroid Treatment Device Market. Governments and private organizations are recognizing the importance of addressing women's health issues, leading to increased funding for research and development in this field. This financial support facilitates the creation of advanced treatment devices and technologies aimed at managing uterine fibroids effectively. As more resources are allocated to women's health, the Uterine Fibroid Treatment Device Market is poised for growth, with new products entering the market that cater to the specific needs of women. Additionally, this investment fosters collaboration between healthcare providers, researchers, and manufacturers, potentially accelerating the development of innovative solutions for uterine fibroid treatment.
Growing Awareness and Education Initiatives
The rise in awareness and education initiatives regarding uterine fibroids significantly impacts the Uterine Fibroid Treatment Device Market. Healthcare organizations and advocacy groups are increasingly focusing on educating women about the symptoms, risks, and treatment options for fibroids. This heightened awareness encourages women to seek medical advice and explore available treatment devices. As a result, the market is likely to experience growth as more women become informed about their health choices. Furthermore, educational campaigns often lead to early diagnosis and intervention, which can improve treatment outcomes. The Uterine Fibroid Treatment Device Market stands to benefit from these initiatives, as they not only empower patients but also drive demand for innovative treatment solutions.
Technological Advancements in Treatment Devices
Technological innovations play a crucial role in shaping the Uterine Fibroid Treatment Device Market. The advent of minimally invasive surgical techniques, such as laparoscopic myomectomy and uterine artery embolization, has revolutionized treatment options for uterine fibroids. These advancements not only reduce recovery times but also minimize complications associated with traditional surgical methods. The integration of robotics and imaging technologies into treatment devices enhances precision and efficacy, appealing to both patients and healthcare providers. As these technologies continue to evolve, the Uterine Fibroid Treatment Device Market is expected to witness significant growth, driven by the demand for safer and more effective treatment modalities. Additionally, the increasing adoption of telemedicine and digital health solutions may further facilitate access to these advanced treatment options.
Rising Demand for Non-Invasive Treatment Options
The demand for non-invasive treatment options is a notable driver in the Uterine Fibroid Treatment Device Market. Patients increasingly prefer treatments that minimize surgical risks and recovery times. Non-invasive procedures, such as MRI-guided focused ultrasound and radiofrequency ablation, are gaining traction as effective alternatives to traditional surgery. This shift in patient preference is likely to influence the market dynamics, as healthcare providers adapt to meet the growing demand for these options. The Uterine Fibroid Treatment Device Market is expected to expand as manufacturers develop and promote non-invasive devices that align with patient desires for safer and more convenient treatment solutions. Furthermore, the increasing success rates of these non-invasive techniques may further bolster their adoption among patients.















Leave a Comment