Rising Incidence of Uterine Fibroids
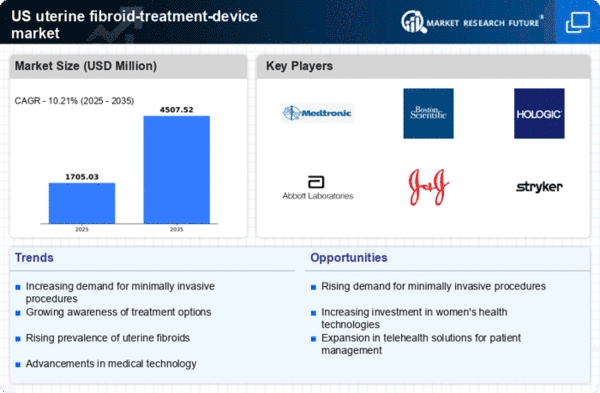
The increasing prevalence of uterine fibroids among women in the US is a primary driver for the uterine fibroid-treatment-device market. Studies indicate that approximately 70-80% of women will develop fibroids by the age of 50. This rising incidence necessitates effective treatment options, thereby propelling demand for innovative devices. As awareness of fibroid-related symptoms grows, more women seek medical intervention, further stimulating market growth. The uterine fibroid-treatment-device market is expected to expand as healthcare providers adopt advanced technologies to address this common condition. The financial implications are notable, with the market projected to reach $2 billion by 2026, reflecting the urgent need for effective solutions to manage fibroid symptoms.
Growing Focus on Women's Health Initiatives
The heightened focus on women's health initiatives in the US is driving the uterine fibroid-treatment-device market. Government and non-profit organizations are increasingly advocating for awareness and education regarding women's health issues, including uterine fibroids. This emphasis on women's health is likely to lead to more funding for research and treatment options, thereby expanding the market. As initiatives promote early detection and treatment, more women are expected to seek medical advice, increasing the demand for effective treatment devices. The uterine fibroid-treatment-device market stands to benefit from this trend, as it aligns with broader efforts to improve healthcare outcomes for women across the nation.
Regulatory Support for Innovative Treatments
Regulatory support for innovative medical treatments is a significant driver for the uterine fibroid-treatment-device market. The US Food and Drug Administration (FDA) has streamlined the approval process for new medical devices, encouraging manufacturers to develop and introduce advanced treatment options for uterine fibroids. This regulatory environment fosters innovation, allowing for quicker access to cutting-edge technologies that can improve patient care. As a result, the uterine fibroid-treatment-device market is likely to see an influx of new products that meet the evolving needs of patients. The potential for expedited approvals could lead to a market growth rate of 10% annually, reflecting the positive impact of regulatory support on device innovation.
Advancements in Minimally Invasive Techniques
The shift towards minimally invasive surgical techniques is significantly influencing the uterine fibroid-treatment-device market. These techniques, which include laparoscopic and robotic-assisted surgeries, offer patients reduced recovery times and lower complication rates compared to traditional surgical methods. As healthcare providers increasingly adopt these advanced technologies, the demand for specialized devices is likely to rise. The market for minimally invasive procedures has shown a growth rate of approximately 15% annually, indicating a robust trend towards less invasive treatment options. This evolution in surgical practices not only enhances patient outcomes but also drives the uterine fibroid-treatment-device market towards innovative solutions that align with patient preferences for less invasive care.
Increased Investment in Healthcare Technology
Investment in healthcare technology is a crucial driver for the uterine fibroid-treatment-device market. With the US healthcare system increasingly prioritizing technological advancements, funding for research and development in medical devices has surged. This influx of capital enables manufacturers to innovate and improve existing treatment devices, enhancing their efficacy and safety. The uterine fibroid-treatment-device market benefits from this trend, as new technologies emerge that cater to the specific needs of patients suffering from fibroids. In 2025, the healthcare technology sector is projected to reach $500 billion, with a significant portion allocated to developing devices for conditions like uterine fibroids, thereby fostering market growth.




















Leave a Comment