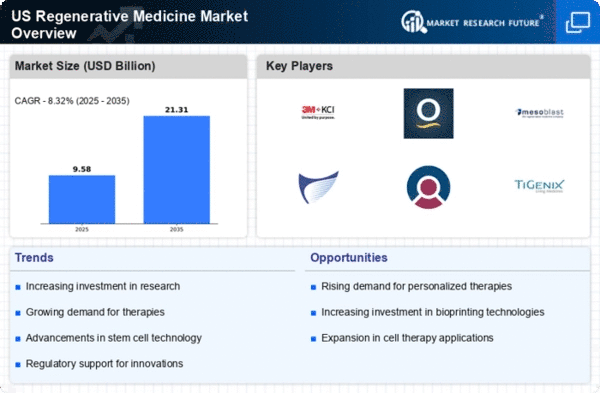
Top Industry Leaders in the US Regenerative Medicine Market

Latest US Regenerative Medicine Companies Update:
First ever gene therapy for Duchenne Muscular Dystrophy: Sarepta Therapeutics received FDA approval for SRP-9001 (ELEVIDYS), a gene therapy treatment for patients with Duchenne Muscular Dystrophy, marking a groundbreaking milestone in gene therapy applications.
Expansion of CAR-T therapy indications: KITE Pharma's Yescarta® received FDA approval for treating follicular lymphoma, opening up possibilities for CAR-T therapy in additional cancer types.
FDA grants Fast Track designation to stem cell therapy for osteoarthritis: Asterias Biotherapeutics secured Fast Track designation for its AST-004, a mesenchymal stem cell therapy candidate for treating knee osteoarthritis, potentially accelerating its development process.
Regenerative Medicine Ventures raises $300 million in Series B funding: This significant investment signifies growing investor confidence in the regenerative medicine market's potential.
Collaboration between CRISPR Therapeutics and Vertex Pharmaceuticals: The companies will jointly develop and commercialize gene editing therapies for sickle cell disease and beta-thalassemia, leveraging their combined expertise.
Establishment of new cell and gene therapy centers: Leading hospitals and research institutions are expanding their capabilities in cell and gene therapy, aiming to provide patients with access to these innovative treatments.
List of US Regenerative Medicine companies in the market
-
Biogen (US)
-
Sarepta Therapeutics, Inc. (US)
-
Gilead Sciences, Inc. (US)
-
Amgen, Inc. (US)
-
Vericel Corporation (US)
-
MiMedx (US)
-
Organogenesis Inc. (US)
-
Medtronic plc (US)
-
BioRestorative Therapies, Inc (US)
-
bluebird bio, Inc. (US)
-
Athersys, Inc. (US)