Market Analysis
In-depth Analysis of US Regenerative Medicine Market Industry Landscape
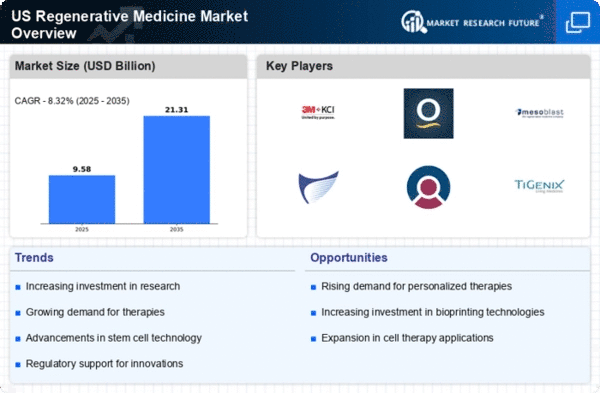
The US regenerative medicine market is encountering dynamic movements driven by mechanical progressions, administrative changes, and expanding interests in innovative work. As of late, regenerative medicine has arisen as a promising field, aiming to furnish the body's innate capacity to repair and recover harmed tissues. Undifferentiated cell therapies, quality therapies, tissue designing, and other inventive methodologies stand out enough to be noticed, adding to the market's dynamism. One critical driver of market elements is the advancing administrative landscape. The US Food and Drug Administration (FDA) has been effectively adjusting its administrative structure to gratify the exceptional difficulties and open doors introduced by regenerative medicine. The presentation of the Regenerative Medicine Advanced Therapy (RMAT) assignment has smoothed out the turn of events and endorsement process for certain therapies, cultivating a more helpful climate for market growth. Administrative rationality and backing have energized both laid out organizations and new companies to investigate and put resources into regenerative medicine. Patient demand and awareness similarly assume an essential part in forming the elements of the regenerative medicine market. As patients progressively look for options in contrast to customary medicines and surgeries, awareness of regenerative medicine's potential advantages has developed. The possibility of customized and regenerative therapies has accumulated interest from patients and healthcare suppliers the same. This expanded demand, combined with a more educated patient populace, fills in as an impetus for market growth and energizes further innovative work endeavors. Challenges inside the market elements incorporate the requirement for standardized assembling processes, versatility of therapies, and long haul security and viability information. Overcoming these difficulties requires progressing joint effort among partners, including industry players, administrative bodies, and scholarly organizations. Also, market players must explore the advancing repayment landscape to guarantee the sustainable commercialization of regenerative medicine items.








Leave a Comment