Rising Demand for Generic Drugs
The US Pharmaceutical Contract Manufacturing Organization Market is experiencing a notable increase in the demand for generic drugs. As healthcare costs continue to rise, both consumers and healthcare providers are seeking more affordable medication options. According to the FDA, the number of approved generic drugs has surged, indicating a shift towards cost-effective alternatives. This trend is likely to drive contract manufacturers to enhance their capabilities in producing generics, thereby expanding their market share. The growing acceptance of generics among physicians and patients further supports this trend, suggesting that contract manufacturers will need to adapt their production processes to meet the increasing demand for high-quality generic pharmaceuticals.
Increased Focus on Supply Chain Resilience
The US Pharmaceutical Contract Manufacturing Organization Market is increasingly prioritizing supply chain resilience. Recent disruptions have highlighted the vulnerabilities within pharmaceutical supply chains, prompting manufacturers to reassess their strategies. By diversifying suppliers and enhancing logistics capabilities, contract manufacturers can mitigate risks associated with supply chain interruptions. This focus on resilience is likely to drive investments in technology and infrastructure, enabling manufacturers to respond more effectively to market fluctuations. As a result, the ability to maintain a stable supply of pharmaceuticals will become a critical factor for success in the competitive landscape of contract manufacturing.
Technological Advancements in Manufacturing Processes
Technological innovations are playing a pivotal role in shaping the US Pharmaceutical Contract Manufacturing Organization Market. The integration of advanced manufacturing technologies, such as continuous manufacturing and automation, is enhancing production efficiency and reducing costs. For instance, the adoption of real-time monitoring systems allows manufacturers to ensure compliance with stringent regulatory standards. As a result, contract manufacturers are likely to invest in these technologies to improve their operational capabilities. The US market is witnessing a shift towards more sophisticated manufacturing processes, which could potentially lead to higher quality products and faster time-to-market for new drugs.
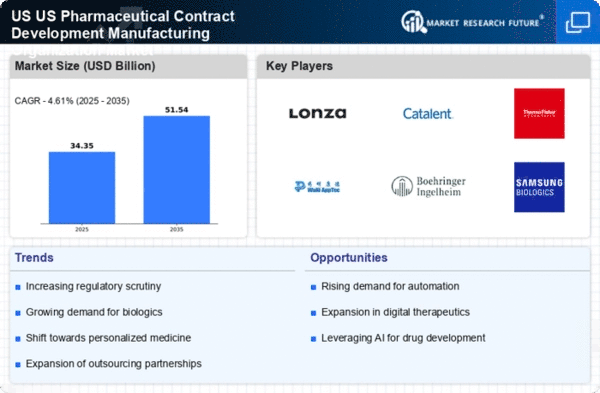
Growing Regulatory Scrutiny and Compliance Requirements
The US Pharmaceutical Contract Manufacturing Organization Market is facing heightened regulatory scrutiny, necessitating strict adherence to compliance requirements. Regulatory bodies, such as the FDA, are implementing more rigorous guidelines to ensure product safety and efficacy. This trend is compelling contract manufacturers to invest in quality assurance and regulatory compliance measures. As a result, companies that can demonstrate their commitment to meeting these standards are likely to gain a competitive edge. The increasing complexity of regulations may also drive consolidation within the industry, as smaller manufacturers may struggle to keep pace with compliance demands.
Expansion of Biopharmaceuticals and Personalized Medicine
The US Pharmaceutical Contract Manufacturing Organization Market is witnessing a significant expansion in the biopharmaceutical sector, driven by the growing demand for personalized medicine. As healthcare shifts towards tailored therapies, contract manufacturers are adapting their capabilities to produce biologics and complex molecules. The market for biopharmaceuticals is projected to grow substantially, with estimates suggesting it could reach over 400 billion USD by 2025. This trend presents lucrative opportunities for contract manufacturers to collaborate with biopharma companies, thereby enhancing their service offerings and market presence. The ability to navigate the complexities of biologics manufacturing will be crucial for success in this evolving landscape.