Rising Incidence of Rare Cancers
The patient derived-xenograft-model market is also influenced by the rising incidence of rare cancers, which often lack effective treatment options. As the understanding of cancer biology deepens, there is a growing recognition of the need for tailored therapeutic approaches. Patient derived-xenograft models provide a unique opportunity to study these rare cancers in a preclinical setting, facilitating the development of targeted therapies. In 2025, it is projected that approximately 200,000 new cases of rare cancers will be diagnosed in the US, underscoring the urgency for innovative research models. This increasing incidence is likely to drive demand for patient derived-xenograft models, as researchers seek to explore novel treatment avenues.
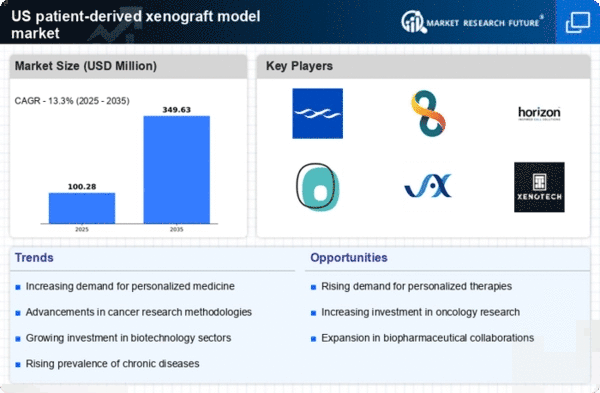
Increasing Investment in Oncology Research
The patient derived-xenograft-model market is experiencing a surge in investment, particularly in oncology research. As the prevalence of cancer continues to rise, funding from both public and private sectors is directed towards innovative research methodologies. In 2025, the National Cancer Institute allocated approximately $6.5 billion to cancer research initiatives, which includes the development of patient derived-xenograft models. This influx of capital is likely to enhance the capabilities of researchers to develop more effective therapies, thereby driving the demand for these models in preclinical testing. The focus on personalized treatment options further emphasizes the need for accurate models that can mimic human tumor biology, making this investment a critical driver in the patient derived-xenograft-model market.
Growing Focus on Drug Development Efficiency
The patient derived-xenograft-model market is being propelled by an increasing emphasis on the efficiency of drug development processes. Pharmaceutical companies are under pressure to reduce the time and costs associated with bringing new drugs to market. Patient derived-xenograft models offer a more predictive platform for evaluating drug efficacy and safety compared to traditional methods. In 2025, it is estimated that the average cost of developing a new drug exceeds $2.6 billion, highlighting the need for more effective preclinical models. By utilizing patient derived-xenograft models, companies can potentially streamline their research and development phases, thereby enhancing their competitive edge in the market. This focus on efficiency is a significant driver for the patient derived-xenograft-model market.
Technological Advancements in Model Development
Technological innovations are playing a pivotal role in the evolution of the patient derived-xenograft-model market. Recent advancements in genetic engineering, imaging techniques, and biomanufacturing processes have significantly improved the efficiency and accuracy of xenograft models. For instance, the integration of CRISPR technology allows for precise genetic modifications, enabling researchers to create models that closely resemble human tumors. This technological progress not only enhances the reliability of preclinical studies but also reduces the time and cost associated with model development. As a result, the patient derived-xenograft-model market is likely to expand, driven by the demand for more sophisticated and representative models in drug discovery and development.
Enhanced Collaboration Between Academia and Industry
The patient derived-xenograft-model market is benefiting from enhanced collaboration between academic institutions and the pharmaceutical industry. Such partnerships are fostering innovation and accelerating the translation of research findings into clinical applications. In 2025, numerous collaborative initiatives are underway, focusing on the development of patient derived-xenograft models for various cancer types. These collaborations often involve sharing resources, expertise, and data, which can lead to more robust model development and validation. As the synergy between academia and industry strengthens, the patient derived-xenograft-model market is likely to see increased growth, driven by the collective efforts to advance cancer research and treatment.