Impact of Aging Population
The aging population in the US is a crucial factor influencing the onychomycosis market. As individuals age, they become more susceptible to various health conditions, including fungal infections of the nails. The demographic shift towards an older population is expected to increase the prevalence of onychomycosis, thereby driving demand for effective treatment options. It is estimated that nearly 50% of individuals over the age of 70 may experience this condition. This demographic trend presents both challenges and opportunities for healthcare providers and pharmaceutical companies. The onychomycosis market must adapt to meet the needs of this growing population segment, which may include developing targeted therapies and enhancing patient education regarding prevention and treatment.
Rising Healthcare Expenditure
The upward trend in healthcare expenditure in the US is a significant driver for the onychomycosis market. Increased spending on healthcare services and pharmaceuticals allows for greater access to advanced treatment options for patients suffering from onychomycosis. As healthcare budgets expand, there is a corresponding rise in the availability of innovative therapies and diagnostic tools. This financial commitment from both public and private sectors is likely to enhance the overall quality of care for patients. Furthermore, the emphasis on preventive healthcare may lead to earlier diagnosis and treatment of onychomycosis, ultimately benefiting the market. The onychomycosis market stands to gain from this trend, as it aligns with broader healthcare initiatives aimed at improving patient outcomes.
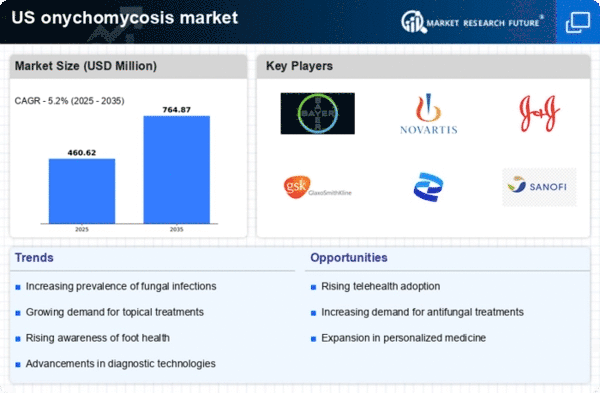
Increasing Prevalence of Onychomycosis
The rising incidence of onychomycosis in the US is a primary driver for the onychomycosis market. Studies indicate that approximately 10% of the adult population is affected by this fungal infection, with the prevalence increasing with age. This growing demographic of affected individuals necessitates effective treatment options, thereby propelling market growth. The onychomycosis market is projected to expand as healthcare providers recognize the need for improved diagnostic and therapeutic solutions. Furthermore, the increasing awareness of foot health and hygiene among the population contributes to the demand for antifungal treatments, which are essential in managing this condition. As the population ages, the burden of onychomycosis is likely to escalate, further driving the market's expansion.
Technological Innovations in Treatment
Technological advancements in the development of antifungal agents and treatment modalities are significantly influencing the onychomycosis market. Recent innovations include the introduction of laser therapy and novel topical formulations that enhance drug delivery and efficacy. These advancements not only improve patient outcomes but also attract investment in research and development, fostering a competitive landscape. The market is witnessing a shift towards more effective and less invasive treatment options, which could potentially increase patient compliance. As a result, the onychomycosis market is expected to benefit from these innovations, with a projected growth rate of around 7% annually over the next few years. This trend indicates a promising future for both patients and manufacturers in the sector.
Growing Demand for Over-the-Counter Treatments
The increasing preference for over-the-counter (OTC) treatments among consumers is shaping the onychomycosis market. Patients are increasingly seeking accessible and convenient solutions for managing their condition without the need for prescription medications. This trend is driven by a combination of factors, including rising healthcare costs and a desire for self-management. The OTC segment is expected to witness substantial growth, with estimates suggesting it could account for over 30% of the total market share by 2026. This shift not only reflects changing consumer behavior but also indicates a potential for increased competition among manufacturers to develop effective OTC antifungal products. Consequently, the onychomycosis market is likely to evolve in response to these consumer preferences.