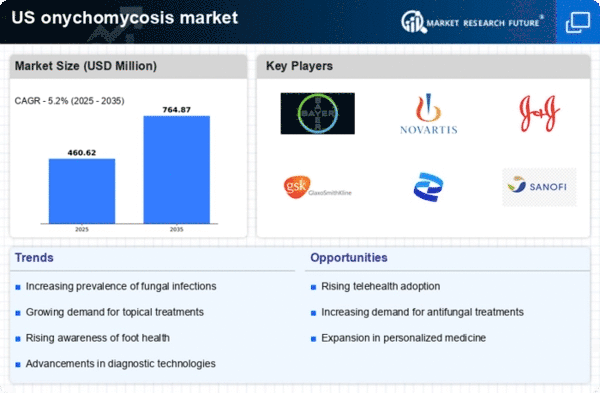
Top Industry Leaders in the US Onychomycosis Market

Kerydin (Tavaborole) Patent Expiry: The key patent for Kerydin, a topical antifungal treatment for onychomycosis, expired in September 2023. This could lead to increased competition and potentially lower prices for patients.
Moberg Pharma Phase 3 Trial: Moberg Pharma, a Danish pharmaceutical company, is conducting a Phase 3 trial for MOB-003, a novel oral therapy for onychomycosis. Positive results could significantly impact the market landscape.
Blueberry Therapeutics Phase 2b Trial: Blueberry Therapeutics is pursuing a Phase 2b trial for its BLU-634 topical gel for onychomycosis. BLU-634 targets a different fungal pathway than existing treatments, offering potential for broader efficacy.
Almirall and Kaken Pharmaceutical Partnership: Almirall, a Spanish pharmaceutical company, entered a partnership with Kaken Pharmaceutical to commercialize Kaken's Luliconazole 5% solution (Luconac R) in the US market. Luconac R is a topical nail lacquer with a unique formulation compared to existing options.
Bausch Health Acquires Valeant Pharmaceuticals: Bausch Health's acquisition of Valeant Pharmaceuticals in May 2023 consolidated its hold on the US onychomycosis market. Valeant previously marketed Jublia (efinaconazole), a topical antifungal treatment.
List of US Onychomycosis Key Companies in the Market
-
Johnson & Johnson
-
Novartis AG
-
Valeant Pharmaceuticals
-
Pfizer Inc.
-
Anacor Pharmaceuticals
-
Galderma S.A.
-
Moberg Pharma AB
-
Bayer AG
-
Dr. Reddy’s Laboratories Ltd.
-
Cipla Ltd.
-
Medimetriks Pharmaceuticals Inc